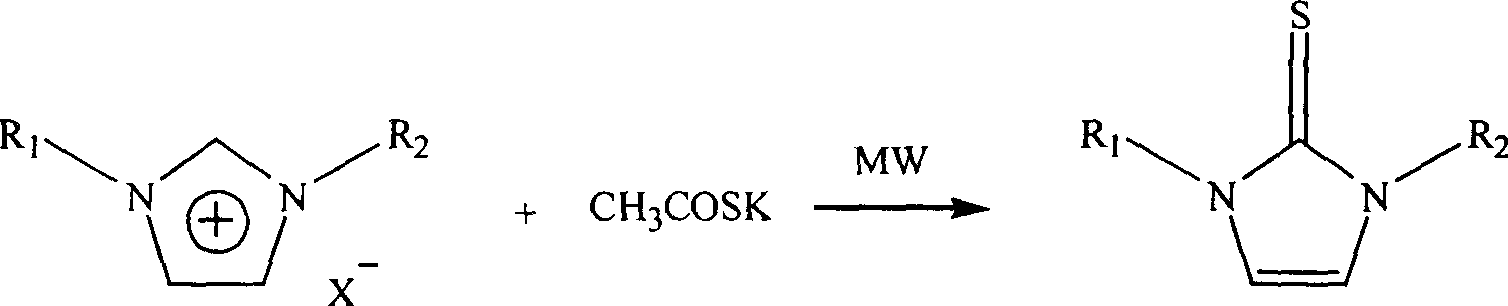Microwave radiation synthesis of 1,3-substituted imidazole-2-thioketone
A technology of disubstituted imidazole and microwave radiation, applied in the direction of organic chemistry, can solve the problems of long reaction time and methanol poisoning, and achieve the effect of simple treatment and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
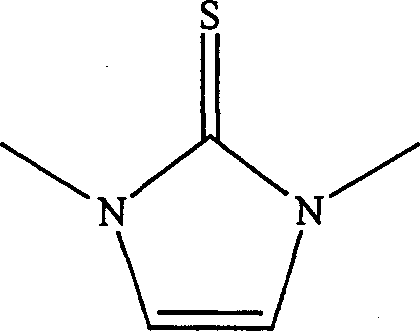
[0013] Example 1 Synthesis of 1,3 dimethylimidazole-2-thione
[0014] Mix 10 mmol 1,3-dimethylimidazole chloride and 11 mmol potassium thioacetate in a 20 ml single-necked bottle evenly, install a reflux condenser, and react under 150W power microwave radiation for 5 minutes, naturally After cooling to room temperature, the reaction mixture was dissolved with 50 ml of ethyl acetate-water (volume ratio 1:1), placed in a separatory funnel, and the organic phase was separated, dried with anhydrous potassium sulfate for 2 hours, concentrated, and washed with silica gel. Column chromatography (eluent: n-hexane: ethyl acetate = 2:1) gave 998 mg of 1,3-dimethylimidazole-2-thione with a yield of 75%.
[0015]
[0016] 1 HNMR (500MHz, CDCl 3 ): δ=3.60(s, 6H), 6.71(s, 2H);
[0017] 13 CNMR (500MHz, CDCl 3 ): δ=34.56, 117.55, 162.12;
[0018] IR (cm -1 )2957, 1445, 1230, 1045;
[0019] MS([M+H] + ): 128.8;
Embodiment 2
[0020] Example 2 Synthesis of 1,3-dimethylimidazole-2-thione
[0021] The reaction steps are the same as in Example 1, except that 1,3-dimethylimidazole bromide is used as the raw material to obtain the product with a yield of 78%.
Embodiment 3
[0022] Example 3 Synthesis of 1,3-dimethylimidazole-2-thione
[0023] The reaction steps are the same as in Example 1, except that 1,3-dimethylimidazolium tetrafluoroborate is used as the raw material to obtain the product with a yield of 79%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com