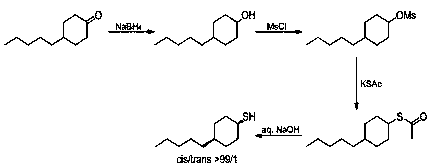
A kind of preparation method of selectively synthesizing cis-4-n-pentyl cyclohexanethiol
A technology of n-pentylcyclohexanone and n-pentyl ring is applied in the field of selective synthesis of cis-4-n-pentylcyclohexanethiol, and can solve the problem of high difficulty in separation and purification, high cost of raw materials and great potential safety hazard. and other problems, to achieve the effect of high cis-product selectivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] first step:
[0018] 22.2g (586.8mmol, 0.3eq) of sodium borohydride and 351.0g of tetrahydrofuran were added to a 2L four-necked flask, and 78.0g (4329.0mmol, 2.2eq) of water was added dropwise under mechanical stirring. After 20 minutes, the temperature of the kettle was basically maintained at 21°C. No significant changes. Then the four-necked bottle was placed in an ice machine and cooled to -5°C for use.
[0019] A solution prepared by 330.0 g of n-pentylcyclohexanone (1961.0 mmol, 1.0 eq) and 171.0 g of tetrahydrofuran was added dropwise, the temperature of the kettle was controlled at -5-5°C, the dropwise addition was completed in 1.5 h, and the stirring was continued for 1.0 h.
[0020] 308.4g (592.0mmol, 0.3eq, 7.0Wt.%) hydrochloric acid was added dropwise to quench the reaction, the temperature of the kettle was controlled at 5-20°C, dichloroethane was added for extraction twice, and the two oil phases were separated and combined. Water was added to wash unti...
Embodiment 2
[0031] first step:
[0032] 2.6g (69.2mmol, 0.3eq) of sodium borohydride and 41.2g of tetrahydrofuran were added to a 250mL four-necked bottle, and 9.1g (507.2mmol, 2.2eq) of water was added dropwise under mechanical stirring. After 20 minutes, the temperature of the kettle was basically maintained at 21°C. No significant changes. Then the four-necked bottle was placed in an ice machine and cooled to -5°C for use.
[0033] A solution prepared by 38.8g (230.6mmol, 1.0eq) of n-pentylcyclohexanone and 19.4g of tetrahydrofuran was added dropwise, the temperature of the kettle was controlled at -5-5°C, the dropwise addition was completed in 0.5h, and the stirring was continued for 1.0h.
[0034] 36.1 g of hydrochloric acid was added dropwise to quench the reaction, the temperature of the kettle was controlled at 5-20° C., dichloroethane was added to extract twice, and the two oil phases were separated and combined. Water was added to wash until neutral, and the oil layer was spin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com