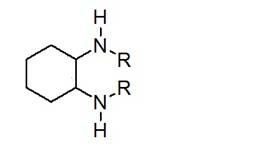
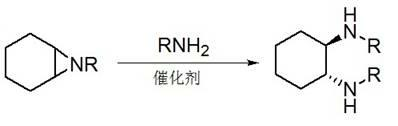
Method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine
A synthesis method and dialkyl technology, applied in the field of chemistry, can solve problems such as manufacturing and cost defects, and achieve the effects of simple operation, cheap and easily available reagents, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of enantiopure N, N'-dimethyl-1,2-cyclohexanediamine
[0038] The first step: 2-methylaminocyclohexanol (IIIa, R=CH 3 ) preparation
[0039] Add epoxycyclohexane (9.8g, 0.1mol) and 25-30% methylamine aqueous solution (18.6g, 0.15mol) into the sealed tube, seal it and heat it to an oil bath temperature of 80°C, and react for 5h. After the system was cooled, the solvent was evaporated under reduced pressure, and the solvent was spin-dried again after adding toluene. After drying, 13 g of 2-methylaminocyclohexanol was obtained, with a yield of 100%;
[0040] The second step: N-methyl-7-azabicyclo[4,1,0]heptane (IVa, R=CH 3 ) preparation
[0041] Add triphenylphosphine (31.44g, 0.12mol) and 2-methylaminocyclohexanol (13g, 0.1mol) into the reaction flask, add solvent CH 2 Cl 2 , slowly add DEAD (22mL, 0.12mol) in CH 2 Cl 2 The solution was raised to room temperature and reacted for 5 hours. After cooling, 2N HCl was added to the system for ext...
Embodiment 2
[0048] Example 2: Preparation of enantiopure N, N'-dimethyl-1,2-cyclohexanediamine
[0049] The first step: 2-methylaminocyclohexanol (IIIa, R=CH 3 ) preparation
[0050] Add epoxycyclohexane (9.8g, 0.1mol) and 25-30% methylamine aqueous solution (18.6g, 0.15mol) into the sealed tube, seal it and heat it to an oil bath temperature of 120°C, and react for 1.5h. After the system was cooled, the solvent was evaporated under reduced pressure, and the solvent was spin-dried again after adding toluene. After drying, 13 g of 2-methylaminocyclohexanol was obtained, with a yield of 100%;
[0051] The second step: N-methyl-7-azabicyclo[4,1,0]heptane (IVa, R=CH 3 ) preparation
[0052] Add triphenylphosphine (31.44g, 0.12mol) and 2-methylaminocyclohexanol (13g, 0.1mol) into the reaction flask, add the solvent THF, slowly add DEAD (22mL, 0.12mol) dropwise under ice bath CH 2 Cl 2 The solution was raised to room temperature and reacted for 6 hours. After cooling, 2N HCl was added to...
Embodiment
[0059] Example 3: Preparation of enantiopure N, N'-diethyl-1,2-cyclohexanediamine
[0060] The first step: 2-ethylaminocyclohexanol (IIIb, R=CH 2 CH 3 ) preparation
[0061] Add epoxycyclohexane (9.8g, 0.1mol) and 70% ethylamine aqueous solution (12.9g, 0.2mol) into the sealed tube, seal it and heat it to an oil bath temperature of 100°C, and react for 3.5h. After the system was cooled, the solvent was evaporated to dryness under reduced pressure, and toluene was added and the solvent was spin-dried again. After drying, 14.3 g of 2-ethylaminocyclohexanol was obtained, with a yield of 100%;
[0062] The second step: N-ethyl-7-azabicyclo[4,1,0]heptane (IVb, R=CH 2 CH 3 ) preparation
[0063] Dissolve triphenylphosphine (31.44g, 0.12mol) and 2-ethylaminocyclohexanol (14.3g, 0.1mol) in tert-butyl methyl ether, and slowly add DIAD (23.8mL, 0.12mol) dropwise under ice-cooling The tert-butyl methyl ether solution was raised to room temperature and reacted for 12 hours. After c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com