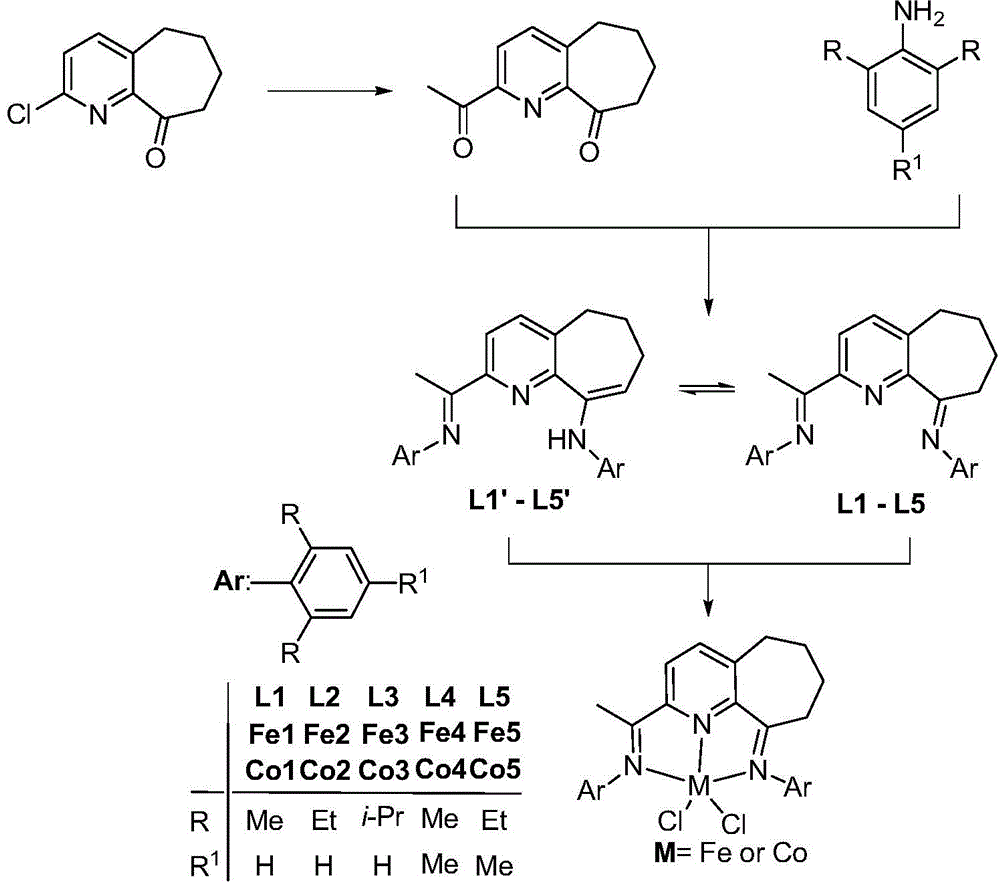
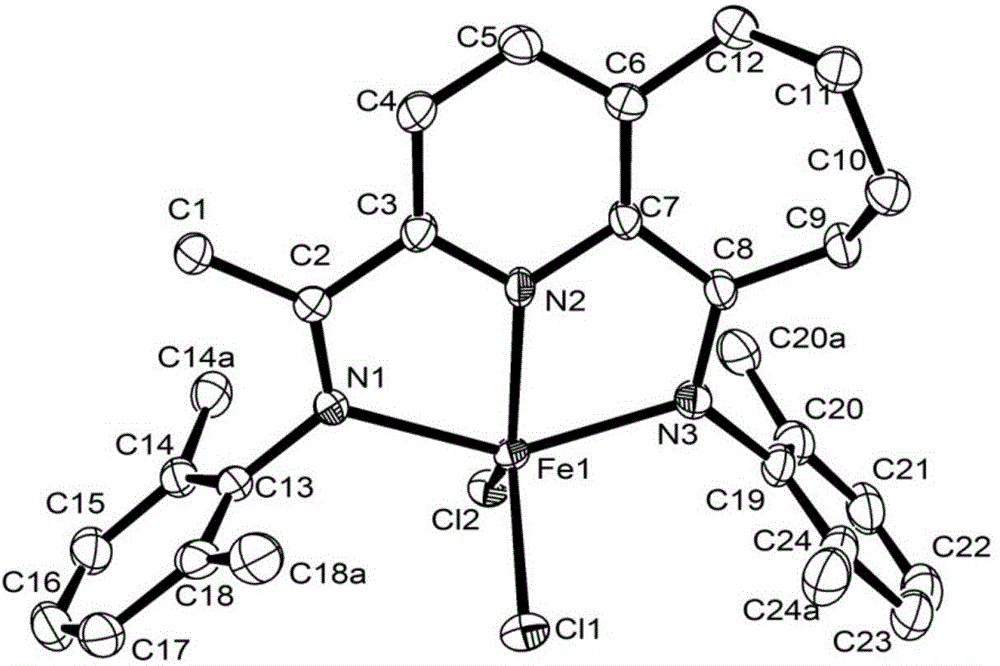
2,6-diimine pyridinocycloheptane iron and cobalt complex catalyst and preparation method therefor and application thereof
A technology of complexes and catalysts, applied in iron organic compounds, nickel organic compounds, organic chemistry, etc., can solve the problems of restricting the development of basic research, poor thermal stability of complexes, and limiting the application research of metal complex catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1. Preparation of (E)-N-(2-((E)-1-(2,6-diisopropylaniline) ethyl)-5,6,7,8-tetrahydrocycloheptane The synthetic method of pyridine-9-dienamine (L3, namely R=i-Pr in the formula V; R 1 = Compound of H):
[0093] 1) Add 2-chloro-5,6,7,8-tetrahydrocycloheptane pyridin-9-one (33.3g, 170mmol, formula II) and 67.7g of tributyl (1-ethyl Oxyvinyl)tin (187mmol), 1,4-dioxane 300ml, catalyst [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride 3.11g (2.5mol%), After 16 hours of reflux, the reaction was complete, and the post-treatment was separated on a silica gel column. The target product was eluted with an eluent whose ratio of eluent was ethyl acetate:petroleum ether=1:50, and 25.9 g of white solid powder was obtained, which was represented by formula III The compound was shown in a yield of 75% (melting point: 88-90°C).
[0094] 2) Add 20 mg of p-toluene to a solution of the compound (0.20 g, 1 mmol) shown in formula III and 2,6-diisopropylaniline (0.39 g, 2.2...
Embodiment 2
[0102] Example 2. Preparation of (E)-N-(2-((E)-1-(2,6-dimethylaniline) ethyl)-5,6,7,8-tetrahydrocycloheptapyridine -the synthetic method of 9-dienamine (L1, namely R=Me among the formula V; R 1 = Compound of H):
[0103] Use the same method as in Example 1 (only 2,6-diisopropylaniline in step 6 described in Example 1 is replaced by 2,6-dimethylaniline) to obtain 0.21g of yellow oil, which is The dienyl amine pyridine ligand compound L1 belonging to formula V (wherein, R is a methyl group, R 1 For hydrogen), the yield is 51.3%.
[0104] The structural confirmation data are as follows:
[0105] 1 HNMR (400MHz, CD 2 Cl 2 ): δ8.36(d, J=8.0Hz, 1H, L1-Py-H), 8.25(d, J=8.0Hz, 1H, L1'-Py-H), 7.64(d, J=8.0Hz, 1H,L1-Py-H),7.52(d,J=8.0Hz,1H,L1'-Py-H),7.04(m,4H,L1-Ar-H),6.98(d,J=7.8Hz, 4H, L1'-Ar-H), 6.90(t, J=7.6Hz, 2H, L1-Ar-H), 6.85(t, J=7.8Hz, 2H, L1'-Ar-H), 6.41(s ,1H,L1'-NH-),4.59(t,J=6.8Hz,1H,L1'-CH=),2.92(t,J=6.0Hz,2H,L1-CH 2 -),2.76(t,J=6.4Hz,2H,L1'-CH 2 -),2.34(s,3H,L...
Embodiment 3
[0110] Example 3. Preparation of (E)-N-(2-((E)-1-(2,6-diethylaniline) ethyl)-5,6,7,8-tetrahydrocycloheptanepyridine -the synthetic method of 9-dienamine (L2, namely R=Et among the formula V; R 1 = Compound of H):
[0111] Use the same method as in Example 1 (only replace 2,6-diisopropylaniline with 2,6-diethylaniline in step 6 described in Example 1) to obtain 0.20 g of a yellow solid, which is the attribution The dienyl amine pyridine ligand compound L2 of formula V (wherein, R is ethyl, R 1 is hydrogen), the yield is 42.9% (melting point: 130-131°C).
[0112] The structural confirmation data are as follows:
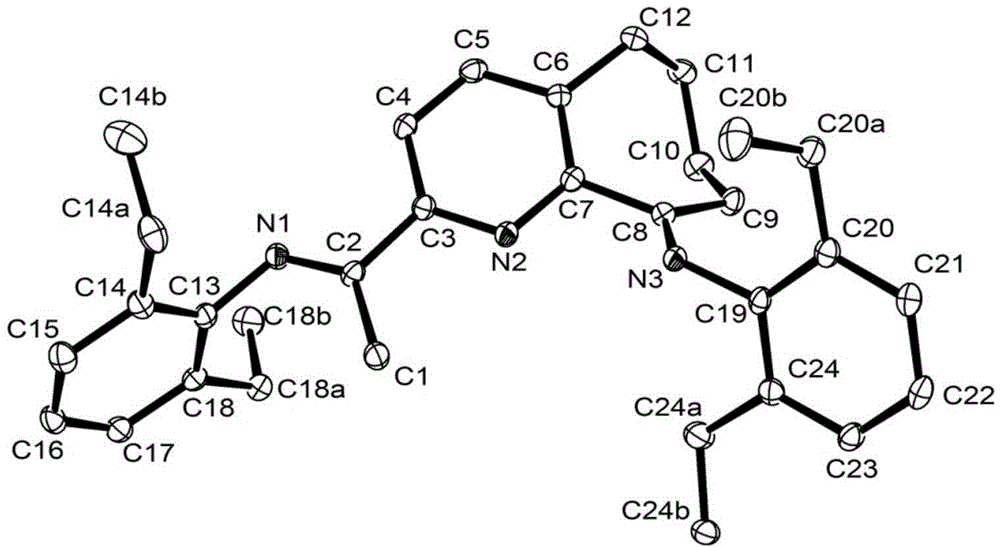
[0113] The schematic diagram of the crystal structure is as figure 2 shown. It can be seen from the figure that the angle between the pyridine ring and the aniline is approximately perpendicular.
[0114] 1 HNMR (400MHz, CD 2 Cl 2): δ8.30(d, J=8.0Hz, 1H, L2-Py-H), 8.20(d, J=8.0Hz, 1H, L2'-Py-H), 7.85(d, J=8.0Hz, 1H, L2-Py-H), 7.61(d, J=8.0Hz, 1H, L2'-Py-H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com