Process for the preparation of cgrp antagonist
A technology of oxo and trifluoroethyl, applied in the field of preparation of CGRP antagonists, can solve the problems of low stability and bioavailability, low yield and optimal yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
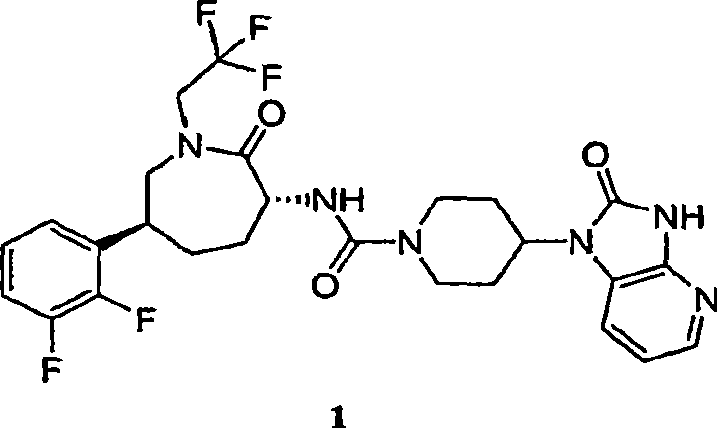
[0081] N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl ]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide
[0082]
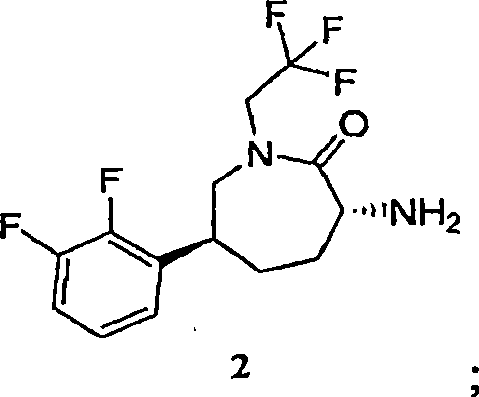
[0083] Into a 12 L 4-neck flask equipped with an overhead stirrer, thermocouple, and nitrogen inlet was charged caprolactam hydrochloride 2-MTBE solvate (412 g corrected for HCl salt; MTBE solvate typically contained 78-79 wt% hydrochloride ). Then, THF (4.1 L; 10 mL / g) was added at room temperature, followed by triethylamine (194 ml; 1.2 equiv). The slurry was aged at room temperature. Into a separate 22L 4-neck flask equipped with an overhead stirrer, thermocouple and nitrogen inlet was charged CDI (233g; 1.25 equiv) and THF (2.3L; 10ml / g relative to CDI). Allow the solution to stand at room temperature. Add the caprolactam slurry solution to the CDI solution over a period of 1 to 1.5 hours at room temperature, then allow it to stand at room temperature for 1 hour, and then assay the reaction to det...
Embodiment 2
[0085] N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl ]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide
[0086]
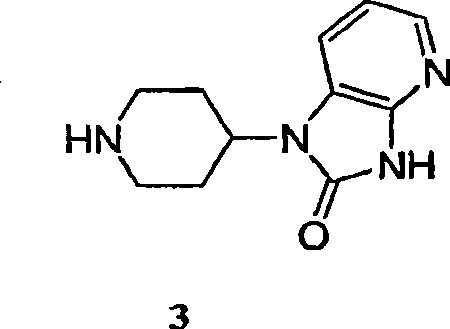
[0087] Caprolactam 2 (8.23 kg (based on 68 wt% assay, 5.60 kg caprolactam hydrochloride)) was charged into inert vessel A with THF (66.4 L) and triethylamine (1.90 kg). Vessel B was charged with CDI (3.163 kg) and THF (30 L). The contents of container A were transferred to container B over a period of 1.5 hours, and the mixture in container B was left for 1 hour. At this point, HPLC analysis indicated that the formation of the caprolactam acylimidazole was nearly complete. Piperidine heterocycle 3 (5.0 kg) was charged into vessel B, followed by triethylamine (4.12 kg). When HPLC analysis indicated that the coupling was complete (3 solution (2 x 28L) for washing. At this point, the pH of the final aqueous phase was 9. The organic phase was washed with DI water (27 L) and the compound was assayed in MT...
Embodiment 3
[0089] N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl ]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide potassium salt ethanolate
[0090]
[0091] A solution of Compound 1 (8.27 kg) in MTBE was charged through a 0.1 μm cartridge filter into an inert vessel and concentrated to 30 L using partial vacuum while maintaining T<40°C. Ethanol (116 L) was added, and the solution was re-concentrated to 30 L under vacuum at <40°C. Ethanol (116 L) was added, and the solution was analyzed for residual THF / MTBE content (not detected). Potassium tert-butoxide (1.720 kg), which would be a solid, was charged to the vessel and the mixture was warmed to 45°C to dissolve all solids. The batch was then concentrated to a final volume of 58 L (based on neutral 454, 7 ml / g) at <40°C. The resulting slurry was allowed to cool to room temperature overnight before filtering. The resulting filter cake was washed with cold ethanol (25 L) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com