Preparation method of loratadine
A technology of loratadine and m-chlorobenzyl chloride, which is applied in the field of drug synthesis, can solve the problems of low yield, difficult separation and purification of products, and high temperature, and achieve the advantages of being beneficial to industrial production, easy to industrial production, and simplifying post-processing steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
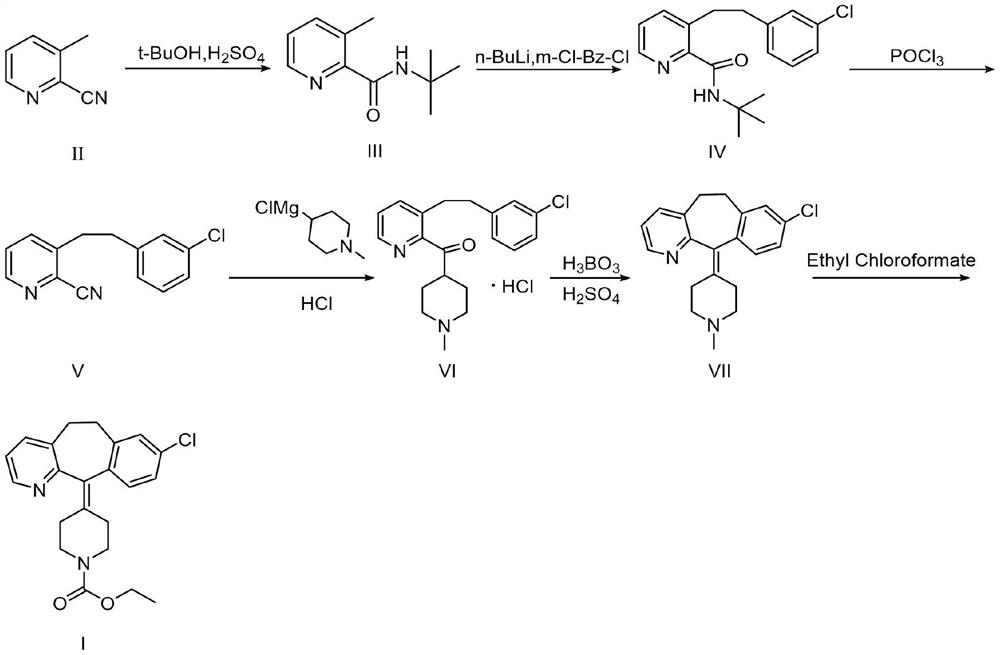
[0044] The preparation method of compound III
[0045] Add tert-butanol (100ml) to the reaction flask, compound II (50.0g, 423.2mmol) is heated to 70°C, after compound II is completely dissolved, slowly add concentrated sulfuric acid (50ml), and the system is heated to 75°C after the dropwise addition After 0.5 h of reaction, TLC detected that the reaction was complete (V ethyl acetate: V petroleum ether = 1:5). Cool to 50-55°C, add water (50ml) and concentrated ammonia water (about 150ml) to adjust the pH to about 9-10, a white solid precipitates, and cool to room temperature. Suction filtration and drying in a blast oven at 25°C for 12 hours gave white crystalline particles (compound III, 70.3 g, 86.5%).
Embodiment 2
[0047] The preparation method of compound IV
[0048]Put compound III (30.0g, 156mmol) in a 1L dry three-neck flask, add freshly distilled tetrahydrofuran (about 450ml) at -10°C under nitrogen protection, cool to -30°C, stir for 10min and then drop slowly Add 2.5mol / L n-butyl lithium (131ml, 327mmol), control the dropping speed, and continue stirring for 10min after the dropping is completed. Then m-chlorobenzyl chloride (22ml, 174mmol) was dissolved in anhydrous tetrahydrofuran (120ml) and added dropwise to the reaction solution. After the dropwise addition, the temperature was kept at -10°C and stirred for 1h. After the reaction was completed, add water (about 100ml) dropwise to quench the reaction. At this time, the red color of the system faded, extracted with EA (150ml×3), anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain a brown-red oily liquid (compound IV, 43.0 g, 87.1%).
Embodiment 3
[0050] The preparation method of compound V
[0051] Phosphorus oxychloride (about 200ml) was added to compound IV (50.0g, 158mmol), and the temperature was raised to 110°C for 4h under reflux. TLC point plate detection reaction is complete (V 乙酸乙酯 :V 石油醚 =1:5), most of the phosphorus oxychloride was distilled off under reduced pressure. After cooling to room temperature, the remaining liquid was poured into ice water (200ml), and 50% sodium hydroxide solution was slowly added under stirring to adjust the pH to 9-10. After the brown solid was precipitated, the stirring was continued for 1 h, and a dark brown solid (34.63 g), stand at room temperature for 2 days. Isopropanol was recrystallized at 55°C (30ml), filtered with suction, and the filter cake was air-dried at 50°C for 12 hours to obtain a light gray solid (Compound V, 21.6g, 50.14%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com