Preparation methods for azlocillin sodium and azlocillin sodium freeze-dried powder for injection
A technology of azlocillin sodium and azlocillin acid, which is applied in the directions of freeze-drying delivery, medical preparations containing active ingredients, and pharmaceutical formulations, can solve the problem that the step control process is not very standardized, the quality of azlocillin sodium is unstable, Insufficient parameter control and other problems, to achieve the effect of improving the safety of clinical use, suitable for promotion, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of azlocillin sodium of the present invention comprises step S1 preparing azlocillin acid and step S2 preparing azlocillin sodium, wherein,
[0044] The step S1 prepares azlocillin acid, comprising the following steps:
[0045] S1-1: Material preparation: 40g of ampicillin trihydrate, 532g of dichloromethane, 11.6g of triethylamine, 16g of imidazole chloride;
[0046] S1-2: Add ampicillin trihydrate and dichloromethane into the reaction vessel, and stir evenly;
[0047] S1-3: Cool the liquid medicine in the reaction vessel to 10-13°C, add triethylamine, and stir until all the solids are dissolved;
[0048] S1-4: Control the temperature of the liquid medicine at 3-5°C, add imidazole chloride, adjust the pH to 7-8.5, and react for 1-1.5 hours;
[0049] S1-5: filtering and extracting the reaction liquid;
[0050] S1-6: Cool to 3-5°C, adjust the pH to 3-4, and precipitate the precipitate;
[0051] S1-7: The precipitate was taken out, washed and dr...
Embodiment 2 Embodiment 3
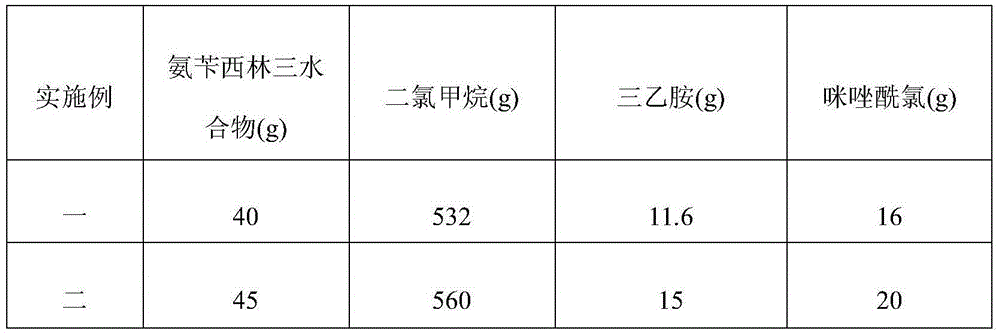
[0069] Referring to Table 1, the difference between Embodiment 2 and Embodiment 3 and Embodiment 1 is that the addition content of ampicillin trihydrate, dichloromethane, triethylamine and imidazole chloride in the material preparation of the step S1-1 is replaced For the parts by weight shown in Table 1, the other steps are the same as in Example 1.
[0070] Table 1 The amount of different components added in the material preparation of step S1-1
[0071]
[0072]
Embodiment 4
[0073] Embodiment four and embodiment five
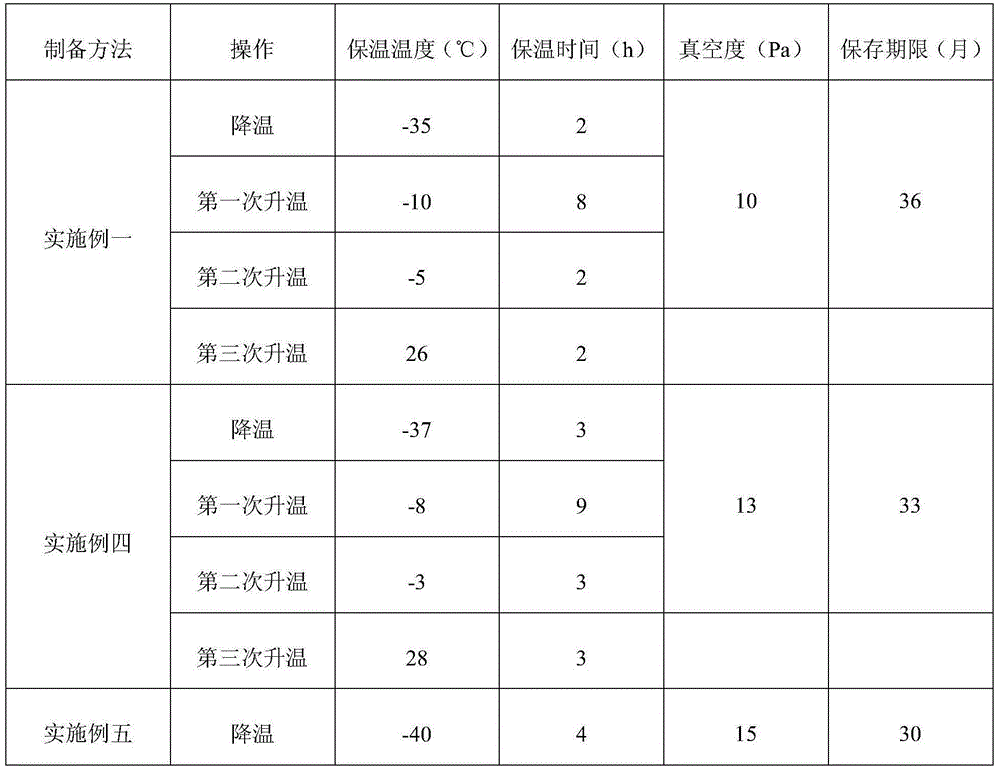
[0074] Referring to Table 2, the difference between Embodiment 4 and Embodiment 5 and Embodiment 1 is that the cooling temperature and heat preservation time in the steps S3-1 to S3-5, the first heating temperature and heat preservation time, the second time The heating temperature and holding time, the third heating temperature and holding time, and the degree of vacuum are replaced by the values shown in Table 2, and the other steps are the same as in Example 1, and the stability study of the resulting finished product is continued at room temperature , the results are as shown in Table 2. Compared with the prior art, the method of the present invention adopts a vacuum low-temperature freeze-drying process to prepare medicines in airtight containers to ensure that the medicines are not easy to oxidize and deteriorate, and overcome the problem of decomposition of medicines caused by high temperatures in the production process. Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com