Azlocillin sodium freeze-dried powder injection for injection prepared from double excipients
A technology for azlocillin sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations and preparations, can solve the problems of low qualification rate of finished product clarity and solution color, high drying temperature, long freeze-drying time, etc., so as to reduce the production of freeze-drying Time, excellent product quality, the effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
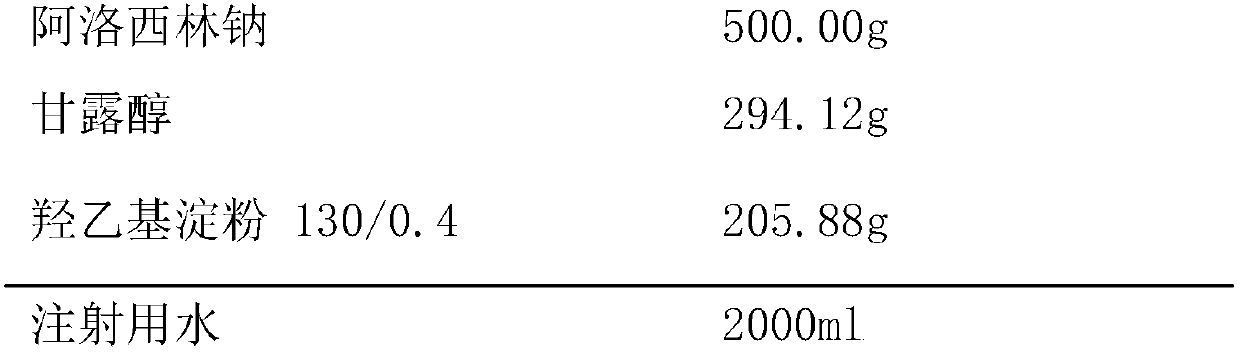
[0024] Embodiment 1, azlocillin sodium freeze-dried powder for injection, calculated in 1000 bottles
[0025] Prescription (specification: 0.5g / bottle):
[0026]
[0027] Preparation Process:
[0028] a) Dissolve the prescribed amount of mannitol in water for injection, place in a constant temperature water bath and stir with a magnetic stirrer at a temperature of 25°C until it is completely dissolved, then add the prescribed amount of hydroxyethyl starch 130 / 0.4 and the prescribed amount respectively Azlocillin sodium, kept at 25°C and continued to stir until completely dissolved, was fixed to 2000ml with water for injection, and the pH value of the solution was adjusted to 6.5.
[0029] b) The above solution is circulated and filtered successively through 0.45 μm and 0.22 μm filter membranes for 30 minutes;
[0030] c) Sampling inspection, determine the filling volume and pour it into a cleaned and sterilized vial, put it into a vacuum freeze dryer after half-tightening...
Embodiment 2
[0031] Embodiment two, azlocillin sodium freeze-dried powder for injection, in 1000 bottles
[0032] Prescription (specification: 0.5g / bottle):
[0033]
[0034] Preparation Process:
[0035] a) Dissolve the prescribed amount of mannitol in water for injection, place in a constant temperature water bath and stir with a magnetic stirrer at a temperature of 25°C until it is completely dissolved, then add the prescribed amount of hydroxyethyl starch 130 / 0.4 and the prescribed amount respectively Azlocillin sodium, kept at 25°C and continued to stir until completely dissolved, was fixed to 2000ml with water for injection, and the pH value of the solution was adjusted to 6.5.
[0036] b) The above solution is circulated and filtered successively through 0.45 μm and 0.22 μm membranes for 30 minutes.
[0037] c) Sampling inspection, determine the filling volume and pour it into a cleaned and sterilized vial, put it into a vacuum freeze dryer after half-tightening, turn on the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com