Preparation method of azlocillin sodium and azlocillin sodium used for injection
A technology of azlocillin sodium and azlocillin acid, which is applied in the direction of organic chemistry, can solve the problems of low reaction efficiency, and achieve the effects of improving reaction efficiency, increasing yield, and being easy to dissolve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
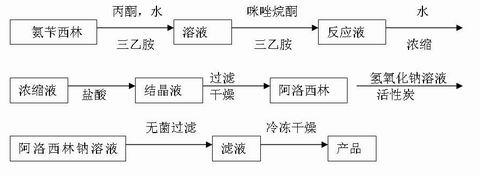
[0044] Embodiment 1 - the preparation of azlocillinic acid.
[0045] 1. Feeding formula:
[0046] Ampicillin Trihydrate 30 Kg
[0047]Acetone 50~55 Kg
[0048] Triethylamine 7.5 Kg
[0049] 1-Chloroformyl-2-imidazolidinone 10.92Kg
[0050] Purified water 240 Kg.
[0051] 2. crafting process:
[0052] Weigh the raw materials according to the above feeding formula, put acetone, purified water, and ampicillin trihydrate into the synthesis tank at about 25°C, and stir evenly. Cool the feed liquid in the synthesis tank. When the temperature is below 15°C, add triethylamine dropwise and stir to make the solution completely clear. Control the temperature of the feed liquid at 6~9°C and slowly add 1-chloroformyl-2-imidazolidinone at a uniform speed, and complete the addition within 25~30 minutes. After feeding, stir for 1 hour, and add 150L (150 Kg). Keep the temperature between 6~9℃, and stir for 2~3 hours. The reaction solution was concentrated, and hydrochloric acid was ...
Embodiment 2
[0053] Embodiment 2---the preparation of azlocillin sodium.
[0054] 1. Feeding formula:
[0055] Azlocillin acid: water for injection 1:3.0~3.5
[0056] Activated carbon for needles: 2.0~4.0% by weight of azlocillinic acid.
[0057] 2. Process:
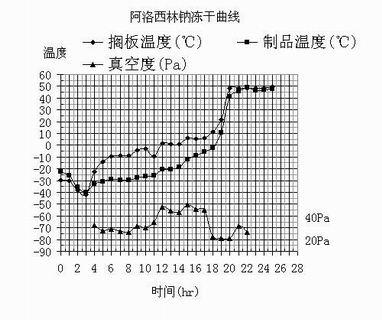
[0058] Add the azlocillin acid prepared according to Example 1 into water for injection, and cool the feed solution to 0-5°C. Add 2M NaOH dropwise to adjust the pH to 7.0~7.5. Add activated carbon for needles, stir for 20 minutes, press filter, and decarbonize through a plate and frame filter. The decarbonized filtrate is sterilized and filtered through 0.45 μm and 0.22 μm filters to a 10,000-class clean area, and placed on a plate under a 100-class laminar flow to a freeze-drying box. The liquid thickness of each plate is about 10mm. When the temperature of the board layer drops below -30°C, the products are packed into boxes. Continue to cool down. After the product temperature reaches -40°C (about 1.5~2h), turn on the vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com