Multimeric protein having effect of brain targeting, and preparation method and usage thereof
A protein and brain-targeted technology, which is applied to peptide/protein components, medical preparations containing active ingredients, chemical instruments and methods, etc., can solve the problems of increased canceration, short time, unstable curative effect, etc., and achieve transformation efficiency High, easy-to-observe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Construction of Engineering Bacteria Encoding CTB Gene
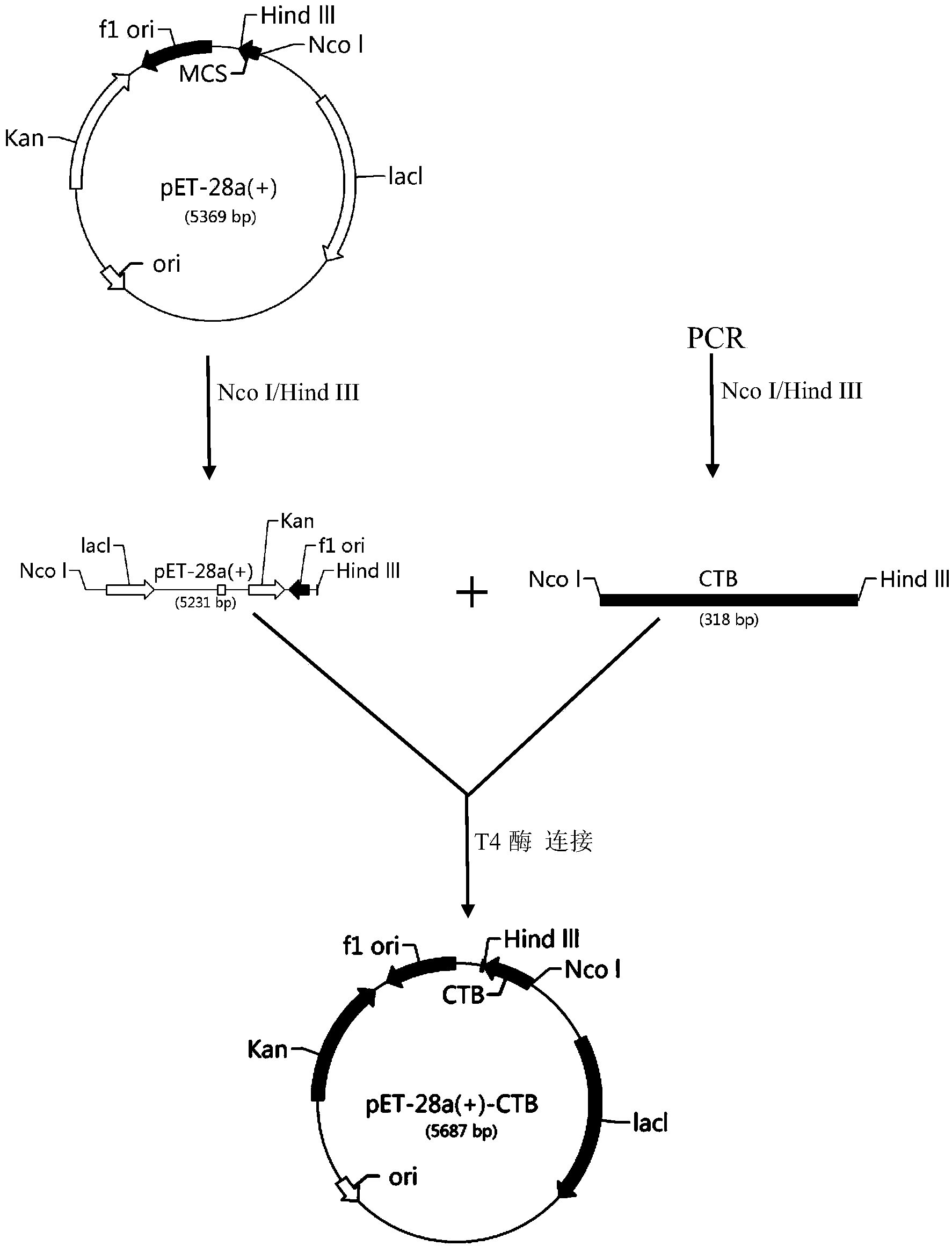
[0060] The natural cholera toxin subunit B consists of 372 nucleotides encoding 124 amino acids. PCR technology is used to remove 63 nucleotides at the front end of subunit B of cholera toxin. These 21 amino acids are the signal peptide of CTB. The expression level of CTB does not affect its biological activity after removal, and its DNA and amino acid sequences are shown in SEQ ID NO: 6 and SEQ ID NO: 2, respectively. The pET-28a-CTB vector construction process is as follows figure 1 shown. The specific steps are as follows:
[0061] 1. Use upstream primer: 5'-CATG CCATGG GAACACCTCAAAATATTACT-3' SEQ ID NO: 8,
[0062] Downstream primer: 5'-CCG CTCGAG ATTTGCCATACTAATTGC-3' SEQ ID NO: 9;
[0063] Carry out PCR amplification, PCR reaction system: 50ul system contains 10×PCR buffer 5ul (excluding Mg2+), dNTP 1ul, upstream and downstream primers 1ul, template DNA 1ul (about 0.7ug), pfu DNA polyme...
Embodiment 2
[0065] Example 2 Construction of Engineering Bacteria Encoding EGFP-CTA2-TAT Gene
[0066] 1. The pET-22b-EGFP-CTA2-TAT vector construction process is as follows Figure 4shown. The specific process is: using the natural amino acid sequences of CTA2, EGFP, and TAT as templates, the gene sequence obtained through codon optimization is shown in SEQ ID NO: 3, which is fully synthesized by the company. The above-mentioned gene sequence is carried out PCR amplification, amplification primer is as shown in SEQ ID NO:10 and SEQ ID NO:11:
[0067] Use upstream primer: 5'-GGGAATTC CATATG GTGAGCAAGG-3' SEQ ID NO: 10
[0068] Downstream primer: 5'-CCG CTCGAG CTGTGGTGGAC-3' SEQ ID NO: 11
[0069] 2. PCR reaction system: 5ul of 10×PCR buffer (without Mg2+) in the 50ul system, 1ul of dNTP, 1ul of upstream and downstream primers, 1ul of template DNA (about 0.5ug), 1ul of pfu DNA polymerase, Mg2+ added to 1.0 mmol / l, add ddH2O to 50ul. The reaction conditions are: pre-...
Embodiment 3
[0071] Example 3 Step-by-step transformation to obtain target protein expression engineering bacteria
[0072] Transform PET-28a-CTB into Escherichia coli BL21(DE3) competent, select stable single colony under 30mmol / lKan antibiotic pressure, induce overnight at 37℃, IPTG final solubility 0.75mmol / l, 200rpm , and select the engineering bacteria with stable expression. Make the above-mentioned engineering bacteria into a competent state, transform the recombinant plasmid PET-22b-EGFP-CTA2-TAT into the competent state, and screen the engineering bacteria that can be stably passed down under the double resistance selection pressure of 100mmol / l Amp and 30mmol / lKan . Plasmid and bacterial liquid PCR identification such as Figure 7 shown. Enzyme digestion identification such as Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com