Q3 sparc deletion mutant and uses thereof
An antibody and carrier technology, applied in the direction of antibodies, medical preparations containing active ingredients, peptide sources, etc., can solve problems such as the decline of renal SPARCmRNA levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
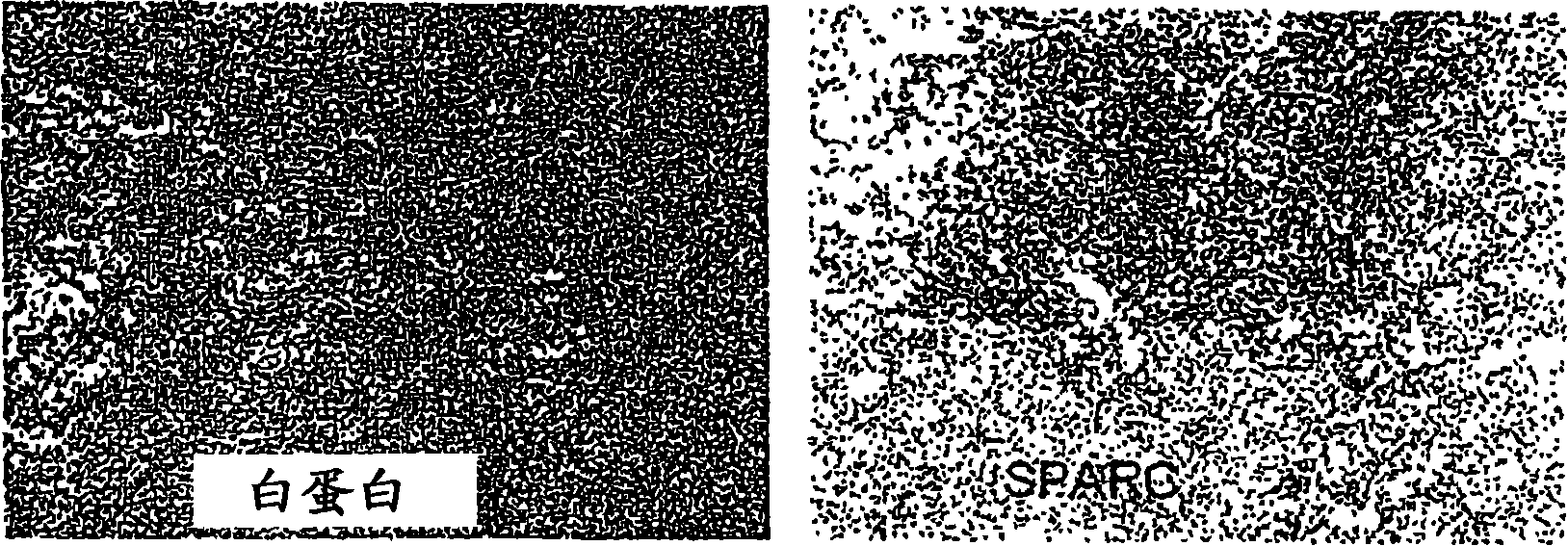
[0164] This example illustrates the co-localization of SPARC and albumin in MX-1 tumor xenografts.
[0165] Paclitaxel albumin nanoparticles (Abraxane, ABX or ABI-007) have been shown to have improved responses over taxol (TAX) in phase 3 metastatic breast cancer trials (33% vs. 19%, p<0.0001) ( See, eg, O'Shaughnessy, SABCS 2003). In the case of ABX versus TAX, albumin-mediated transendothelial transport of paclitaxel (P) and enhanced intratumoral accumulation of paclitaxel were recently demonstrated (see, eg, Desai, SABCS 2003). Albumin binds to SPARC (see, eg, Schnitzer, J. Biol. Chem. 269:6072-82 (1994)).
[0166] The MX-1 tumor cell line is derived from human breast cancer. Serial cryosections of human MX-1 tumor xenografts, human primary breast tumor tissue (n=141) and normal human breast tissue (n=115) were immunostained for albumin, SPARC (using anti-SPARC antibody) and kiln-1 staining were scored (0-4). Cultured MX-1 cells were also immunostained for SPARC. Pacli...
Embodiment 2
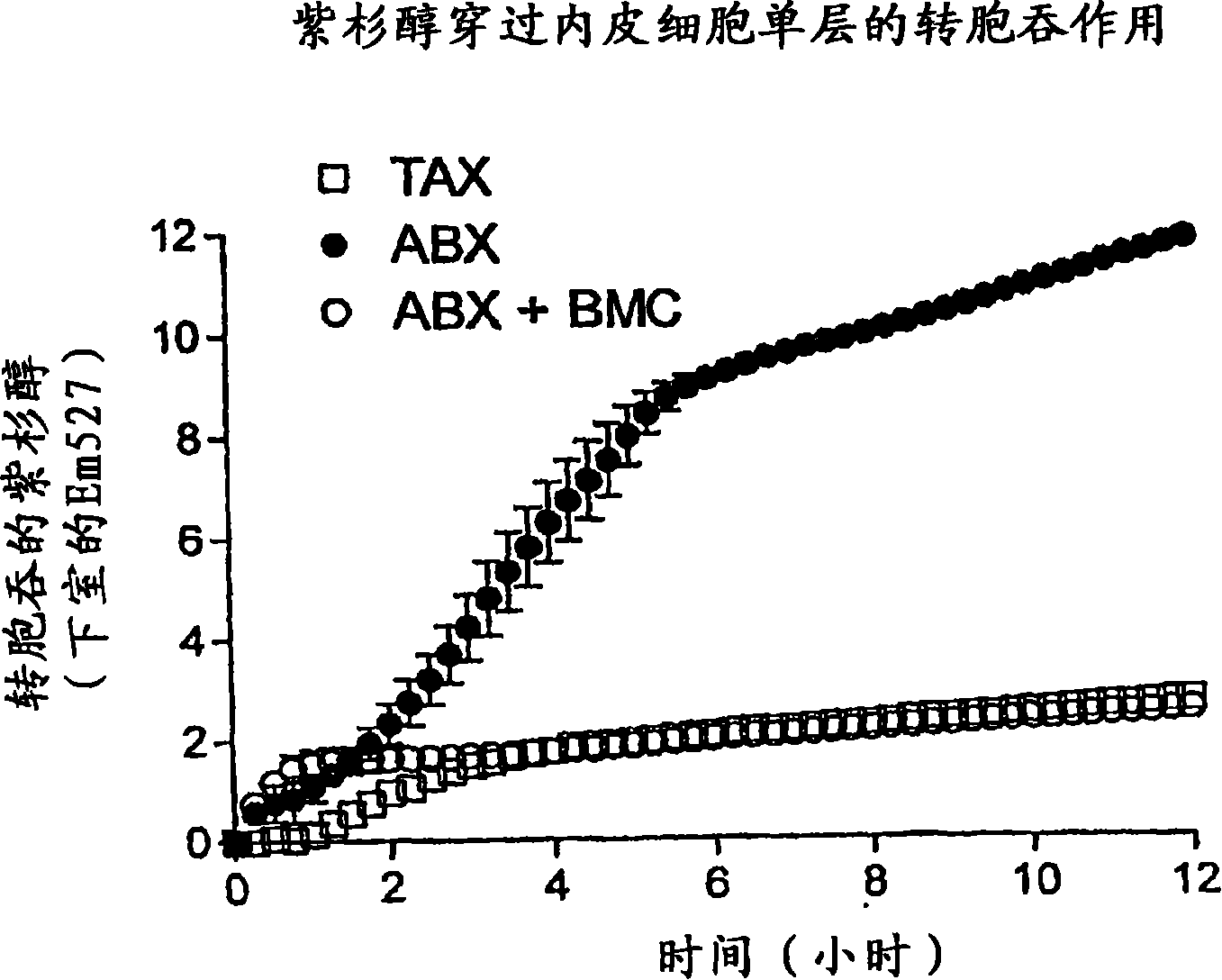
[0170] This example illustrates the endothelial receptor (gp60)-mediated transcytosis of paclitaxel albumin nanoparticles (ABI-007) into caveolae.
[0171] Paclitaxel (P) albumin nanoparticles (Abraxane, ABX, or ABI-007) demonstrated an improved response rate over Taxol in a phase III metastatic breast cancer trial (33% vs. 19%, p<0.0001) (SABCS, O 'Shaughnessy et al., 2003). Cremophor in Taxol (TAX) traps P in micelles in plasma, reducing paclitaxel available for cellular partitioning (see, eg, Sparreboom et al., Cancer. Res. 59, 1454 (1999)). Studies in athymic mice have shown that intratumoral paclitaxel concentrations are 30-40% higher with ABX compared to equivalent doses of TAX (SABCS, Desai et al., 2003). Albumin is transported across endothelial cells (EC) by specific receptor (gp60)-mediated caveolae transport (see, eg, John et al., Am. J. Physiol. 284, L187 (2001)). It was hypothesized that albumin-bound paclitaxel in ABX could be transported across tumor microvasc...
Embodiment 3
[0176] This example illustrates the expression of surface SPARC in MX-1 tumor cells.
[0177] MX-1 cells were grown on coverslips and stained with an antibody against human SPARC using methods known in the art. Observation of antibody staining indicated that MX-1 was expressing SPARC. These results suggest that the SPARC expression detected in MX-1 tumor cells is a consequence of SPARC secretion by MX-1 tumor cells. The staining was more intense for MX-1 tumor cells than for normal primary cells such as HUVEC (human umbilical vein endothelial cells), HLMVEC (human lung microvascular endothelial cells) and HMEC (human mammary epithelial cells). Although the majority of SPARC staining was internal SPARC, significant levels of surface SPARC were also detected, as indicated by confocal microscopy and staining of non-permeabilized cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com