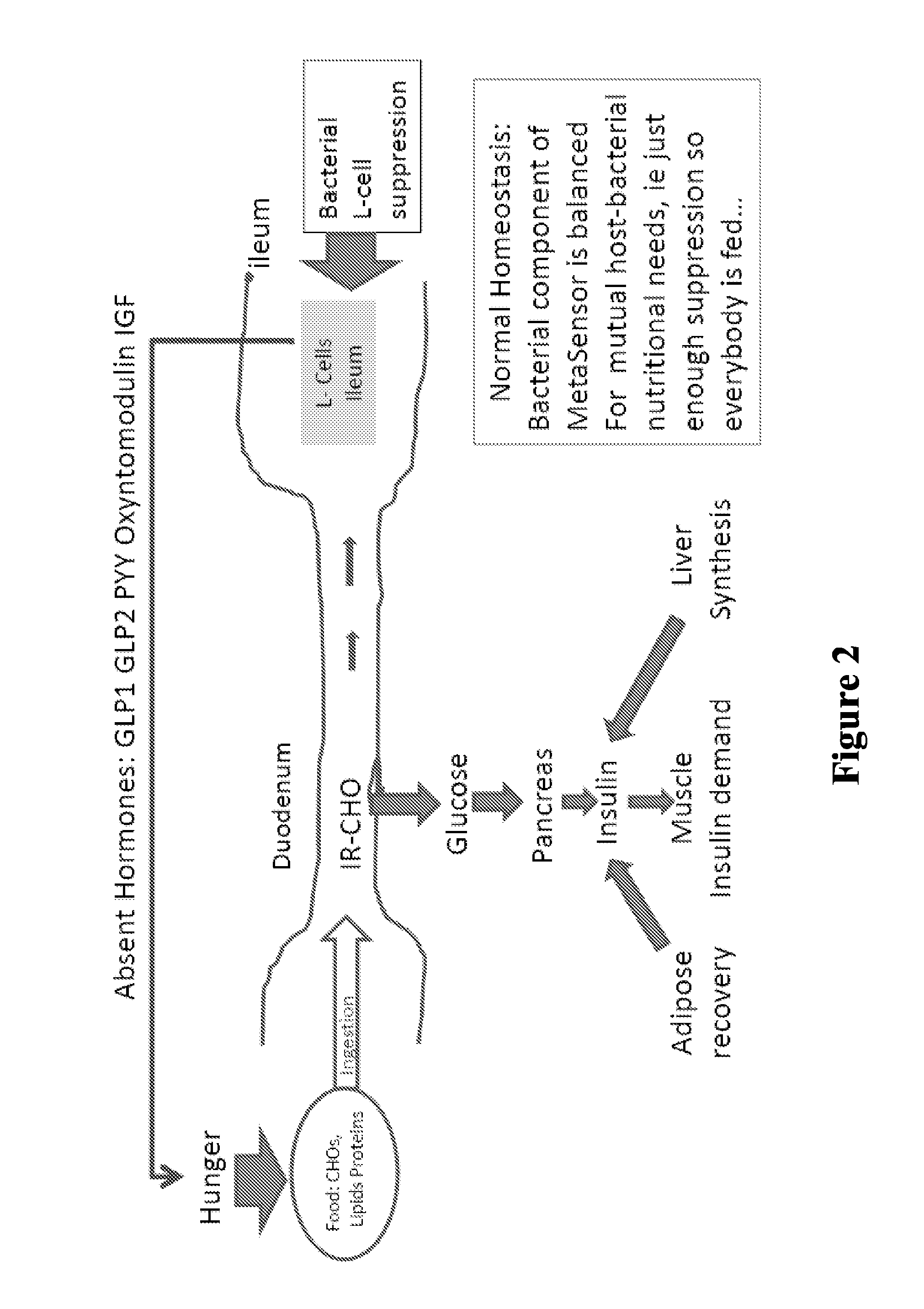
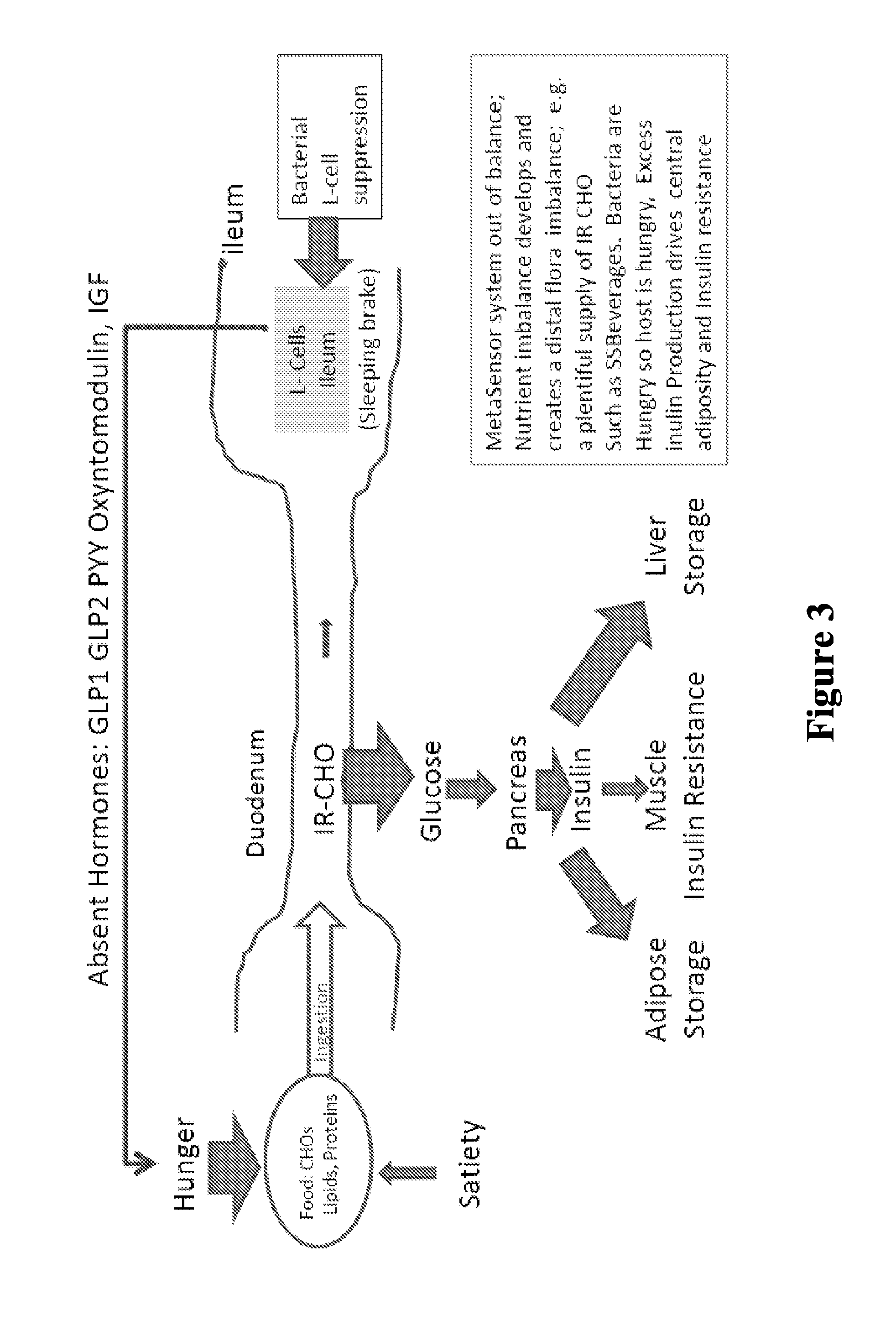
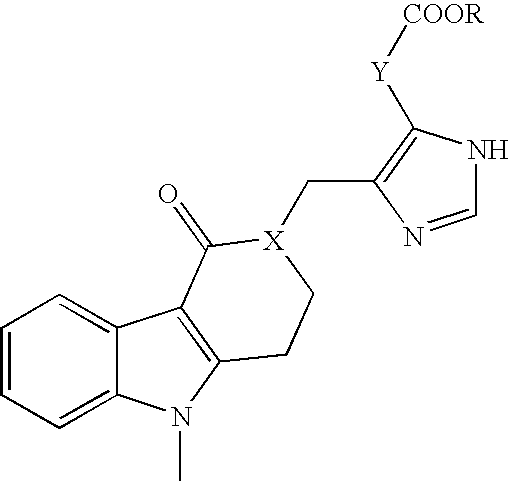
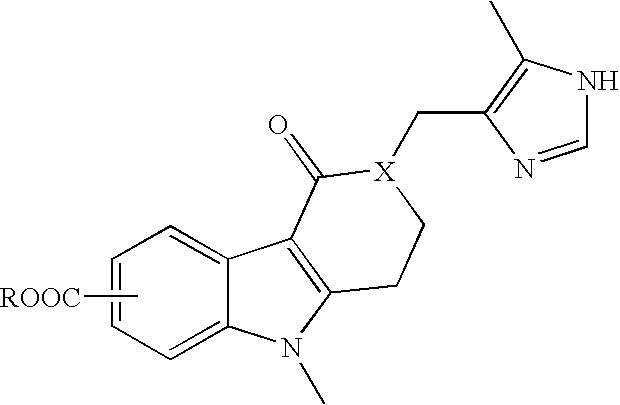
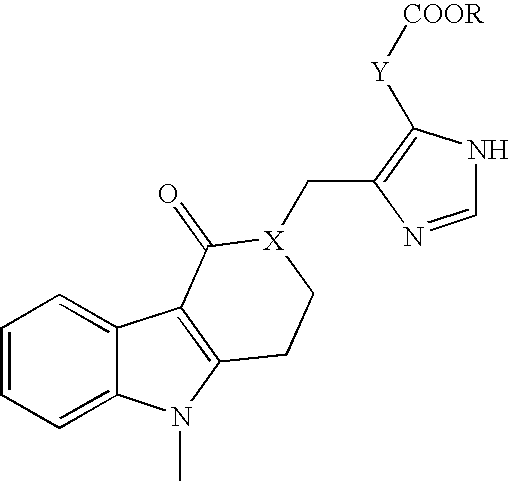
Patents
Literature
169 results about "IBS - Irritable bowel syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Irritable bowel syndrome (IBS) is a group of symptoms that occur together, including repeated pain in your abdomen and changes in your bowel movements, which may be diarrhea, constipation, or both.
Muscarinic receptor antagonists
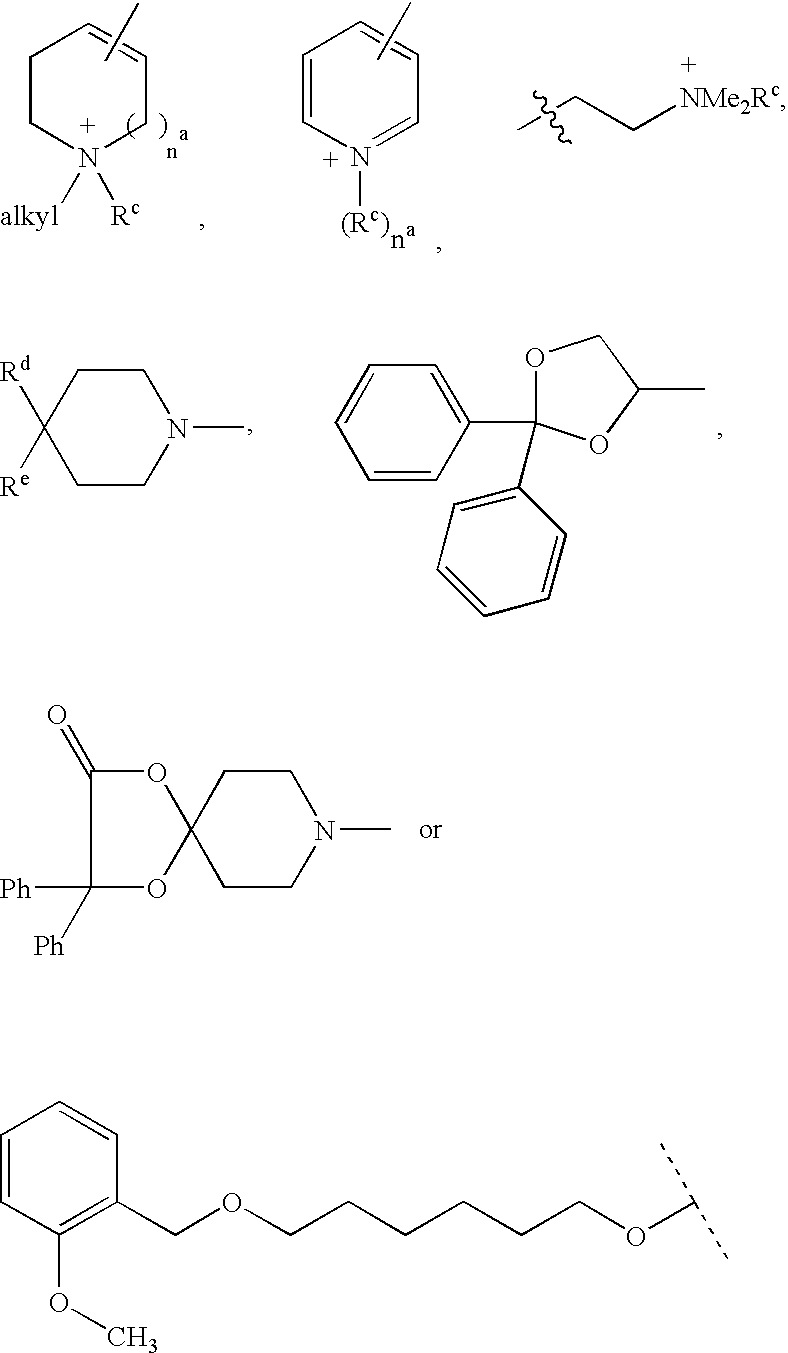
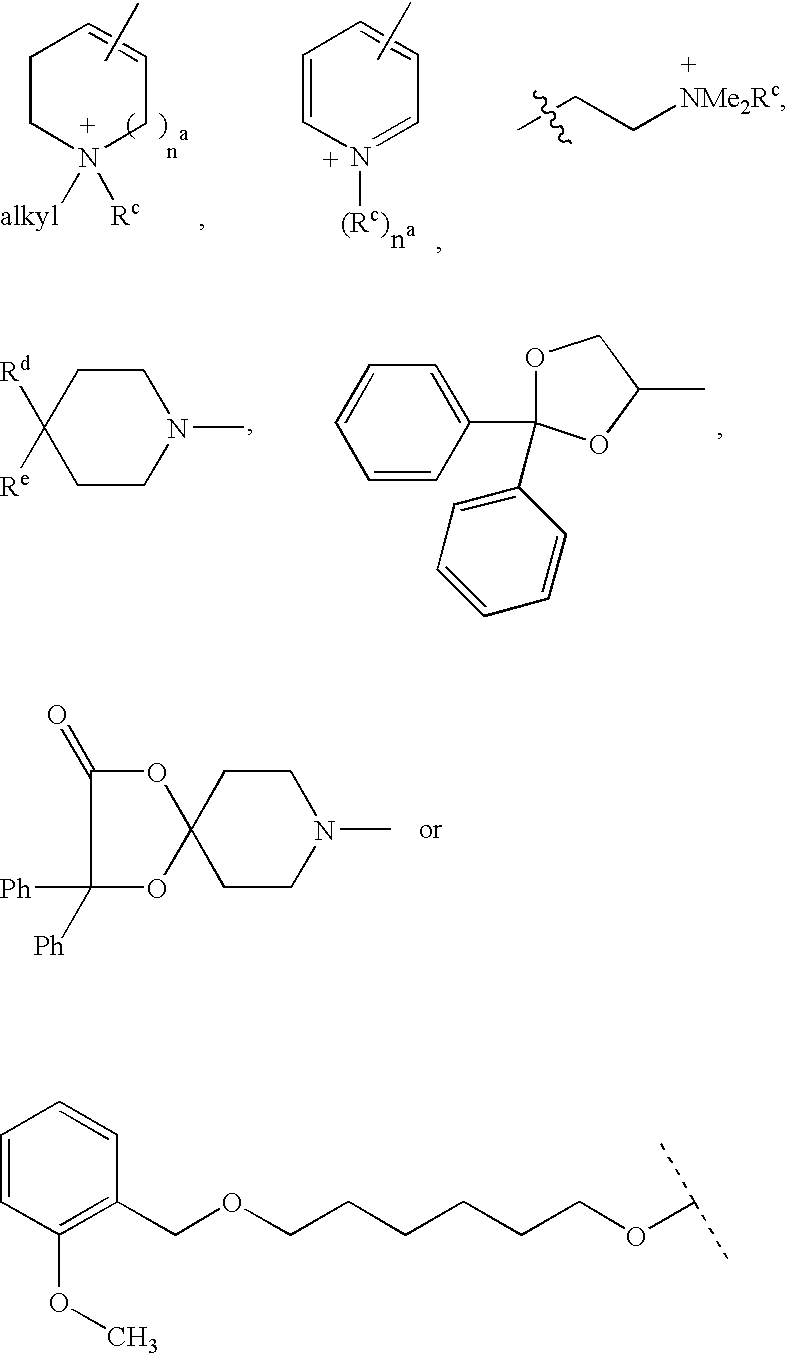
Disclosed are multibinding compounds which are muscarinic receptor antagonists. The multibinding compounds of this invention containing from 2 to 10 ligands covalently attached to one or more linkers. Each ligand is, independently of each other, a muscarinic receptor antagonist or an allosteric modulator provided that at least one of said ligand is a muscarinic receptor antagonist. The multibinding compounds of this invention are useful in the treatment and prevention of diseases such as chronic obstructive pulmonary disease, chronic bronchitis, irritable bowel syndrome, urinary incontinence, and the like.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Use of methylnaltrexone to treat irritable bowel syndrome
Methods of treating irritable bowel syndrome with peripheral opioid antagonists, such as methylnaltrexone, are provided. Formulations comprising peripheral opioid antagonists, such as methylnaltrexone, and irritable bowel syndrome therapeutic agents are also provided.
Owner:PROGENICS PHARMA INC
Method for treatment of disorders of the gastrointestinal system
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Administering osmotic colonic evacuant containing a picosulfate
Colonic evacuation, treatment of small bowel bacterial overgrowth or irritable bowel syndrome or treating acute or chronic bacterial bowel infection comprises administering an osmotic colonic evacuant in solid form, preferably a mixture of sodium dihydrogen phosphate and disodium hydrogen phosphate or a sulfate based laxative comprising a picosulfate, together with a diluent.
Owner:REDHILL BIOPHARMA +1
Method for treating cachexia with retinoid ligands
InactiveUS20070185055A1BiocideSilicon compound active ingredientsRetinoidObstructive Pulmonary Diseases
The present invention relates to a method of treatment of cachexia in a subject in need of treatment. More specifically, the present invention relates to the use of retinoid compounds that act on retinoid X receptors (RXRs) for the treatment of cachexia in a subject in need of treatment. The cachexia is associated with, in other words a complication of, a primary disease, condition or disorder. Primary diseases, conditions and disorders include, but are not limited to, cancer, AIDS, liver cirrhosis, diabetes mellitus, chronic renal failure, chronic obstructive pulmonary disease, chronic cardiac failure, immune system diseases (e.g., rheumatoid arthritis and systemic lupus erythematosus), tuberculosis, cystic fibrosis, gastrointestinal disorders (e.g., irritable bowel syndrome and inflammatory bowel disease), Parkinson's disease, anorexia nervosa, dementia, major depression, an aged condition and sarcopenia.
Owner:JIANG GUANG LIANG +2
Agonists of Guanylate Cyclase Useful for the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders
ActiveUS20100069306A1Maintain good propertiesIncrease resistancePeptide/protein ingredientsAntipyreticPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS7910568B2Easily reach colonPromote absorptionBiocideAntipyreticSalicylic acidBULK ACTIVE INGREDIENT
Owner:ANTIBE THERAPEUTICS INC
Administering osmotic colonic evacuant containing a picosulfate
Colonic evacuation, treatment of small bowel bacterial overgrowth or irritable bowel syndrome or treating acute or chronic bacterial bowel infection comprises administering an osmotic colonic evacuant in solid form, preferably a mixture of sodium dihydrogen phosphate and disodium hydrogen phosphate or a sulfate based laxative comprising a picosulfate, together with a diluent.
Owner:REDHILL BIOPHARMA +1
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Lactobacillus reuteri and its application
ActiveCN107523526AImprove the level ofImprove triglyceridesMilk preparationNervous disorderDiseaseGut flora
The invention relates to the technical field of microorganism, and discloses a Lactobacillus reuteri and its application. The preservation number of the Lactobacillus reuteri CCFM8631 is CGMCC No.14394; the level of mice peripheral neurotransmitter 5-hydroxytryptamine can be significantlyimproved; the rise of the mice peripheral blood testonsterone level and the abundant abnormity of Blautia, Turicibacter, Oscillospira and Bifidobacterium in the intestinal flora by high glucose and high fatty diets are recovered; the tolerated simulative gastrointestinal fluid is rapidly planted in an intestinal tract, so as to significantly improve the pathological injury of metabolic syndrome mice liver and duodenum and rise of triglyceride and total cholesterol content in the serum by high glucose and high fatty diets are significantly improved; the Lactobacillus reuteri can be used for preventing, delaying or treating metabolic disorder such as metabolic syndrome, irritable bowel syndrome, and anxiety, depression and other metal diseases related to irritable bowel syndrome.
Owner:INFINITUS (CHINA) CO LTD
Therapeutic agent for intestinal diseases and visceral pain
InactiveUS20050148632A1Receptor selectivity is unclearImprove securityBiocideAntipyreticDisease irritable bowelVisceral pain
The present invention relates to a therapeutic agent for irritable bowel syndrome of diarrhea type, ulcerative colitis, visceral pain or abdominal pain, which contains a compound of the following formula and which has 5-HT7 receptor antagonistic effect or an analogue thereof; and this therapeutic agent has an excellent therapeutic effect and a high safety:
Owner:AJINOMOTO CO INC
Method and System for Monitoring Gastrointestinal Function and Physiological Characteristics
InactiveUS20080306355A1Efficiently employedPerson identificationAuscultation instrumentsUlcerative colitisAcoustic energy
A system and method for evaluating gastrointestinal motility and, optionally, other physiological characteristics (e.g., pulse rate) that can be effectively employed to acquire one or more signals associated with acoustic energy (i.e. sound) emanating from an abdominal region of a body and determine at least one gastrointestinal parameter or event based on the acoustic energy signal(s) is described. The gastrointestinal parameter can include a gastrointestinal event, including gastrointestinal mixing, emptying, contraction and propulsion, and gastrointestinal transit time, or a gastrointestinal system disorder, including reflux disease, irritable bowel disease, ulcerative colitis, constipation, diarrhea, and a migrating motor complex disorder.
Owner:SMITHKLINE BECKMAN CORP
Method for treating irritable bowel syndrome and other functional gastrointestinal disorders
The invention relates to methods and compositions for the treatment of irritable bowel syndrome (IBS) and other functional gastrointestinal disorders. The methods of the invention involve the administration, in a delayed-release capsule or tablet containing a mixture of peppermint oil and chlorophyll. Other ingredients that may also be effective treatments may be included. This method may be useful as a new and safer treatment for IBS and other functional gastrointestinal disorders. The action of the peppermint oil is effective as a modulator of gastrointestinal motility and sensation. Chlorophyll may improve bowel activity by stimulation of secretion and motility. This is the first description of this unique mixture of these natural products for the treatment of gastrointestinal conditions.
Owner:EHRENPREIS BEN Z +1
Methods of use and nutritional compositions of touchi extract
InactiveUS20090148545A1Reduce complicationsReduce post-prandial glucose excursionBiocideAntiviralsPhysiologyConstipation
Disclosed is a method and composition for nutritional compositions containing -glucosidase inhibitors, and more specifically Touchi Extract and its uses in the treatment of many disorders. These disorders include diabetes, hyperlipidemia, obesity, Metabolic syndrome / Syndrome X, COPD, malabsorption, Crohn's disease, diarrhea, constipation, irritable bowel syndrome, human immunodeficiency virus, cystic fibrosis, non-alcoholic steatohepatitis, polycystic ovarian syndrome including associate infertility, and erectile dysfunction. Further, -glucosidase inhibitors, and more specifically Touchi Extract can be used to aid healing in critical care patients and for general wound healing. Additionally, -glucosidase inhibitors, including Touchi Extract can be used to enhance athletic performance.
Owner:NESTEC SA
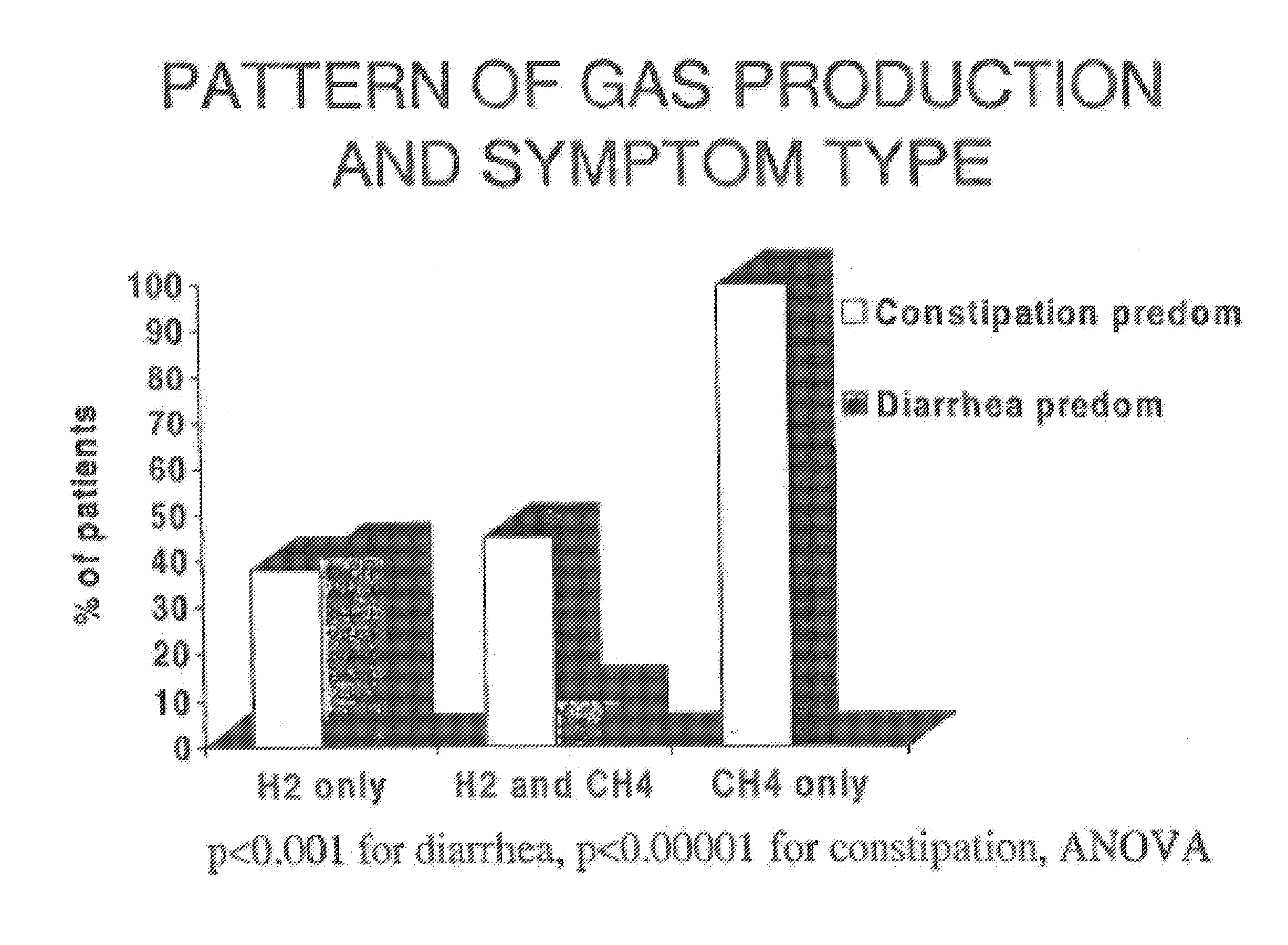
Diagnosis of constipation by analysis of methane concentration
ActiveUS20080182291A1Microbiological testing/measurementDisease diagnosisConstipation predominant irritable bowel syndromeMedicine
Disclosed are methods of diagnosing constipation and constipation-predominant irritable bowel syndrome by screening for the presence of abnormally high levels of methane and / or methanogenic organisms in a subject.
Owner:CEDARS SINAI MEDICAL CENT
Therapeutic agents useful for treating pain
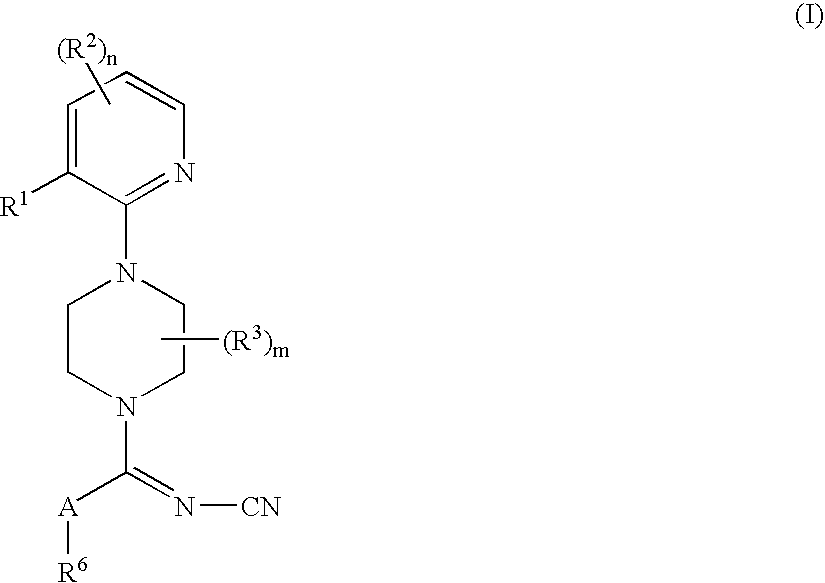
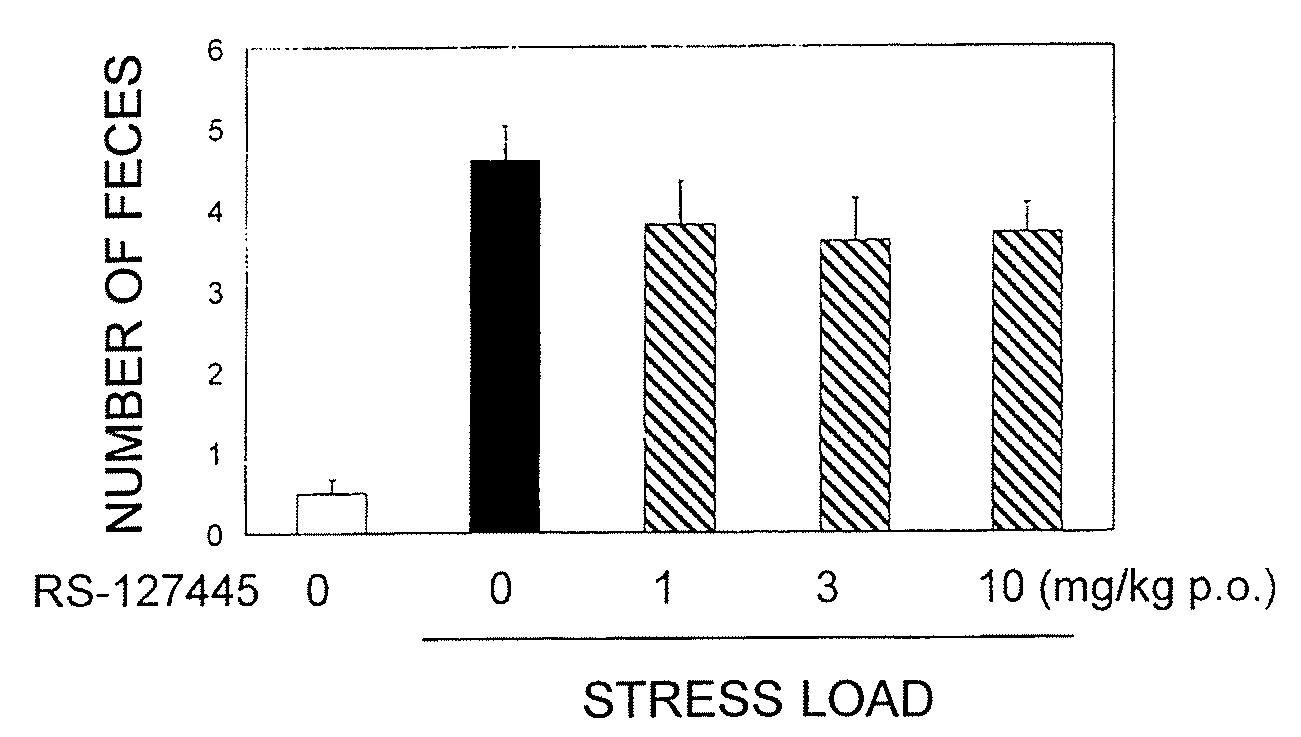
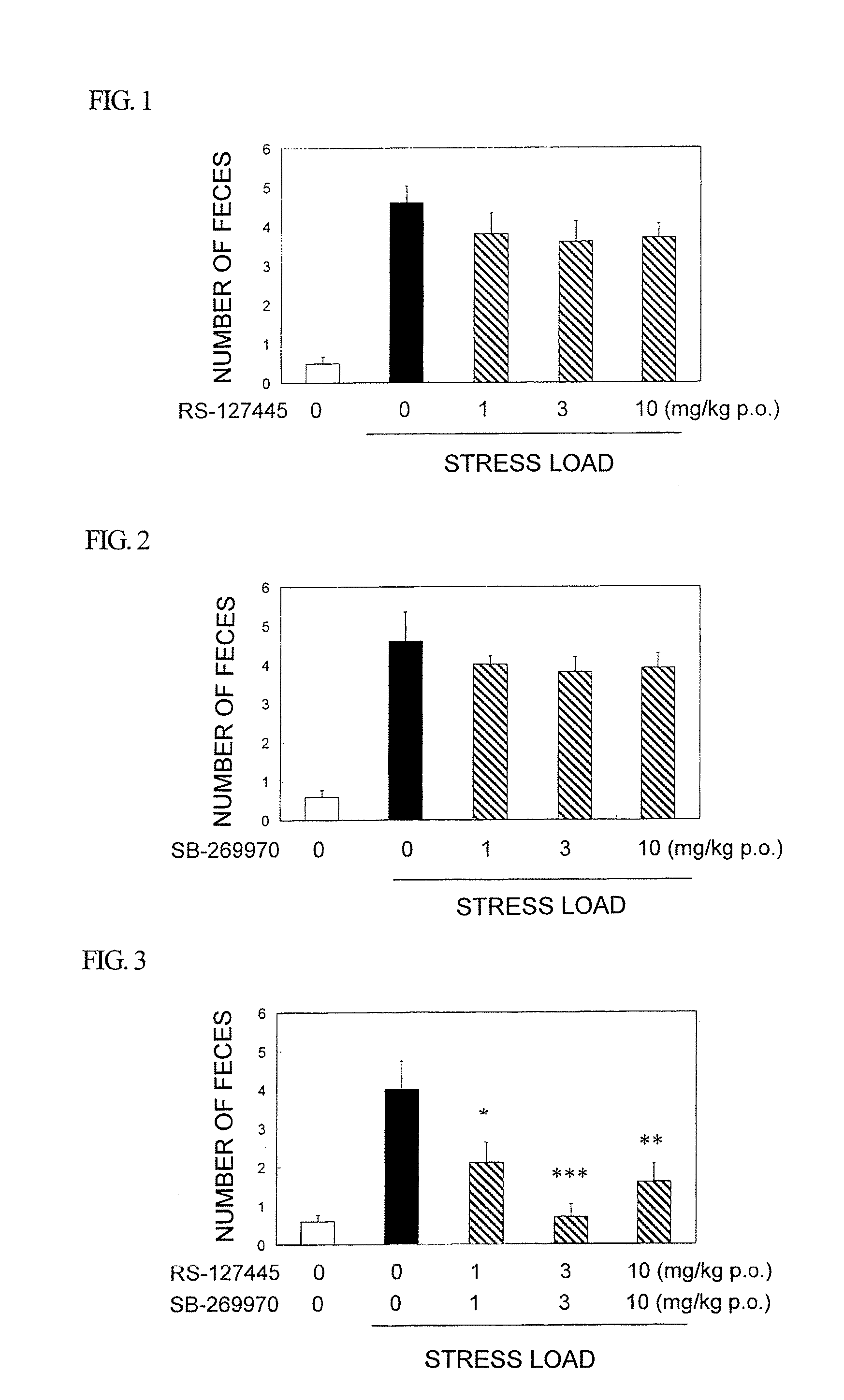
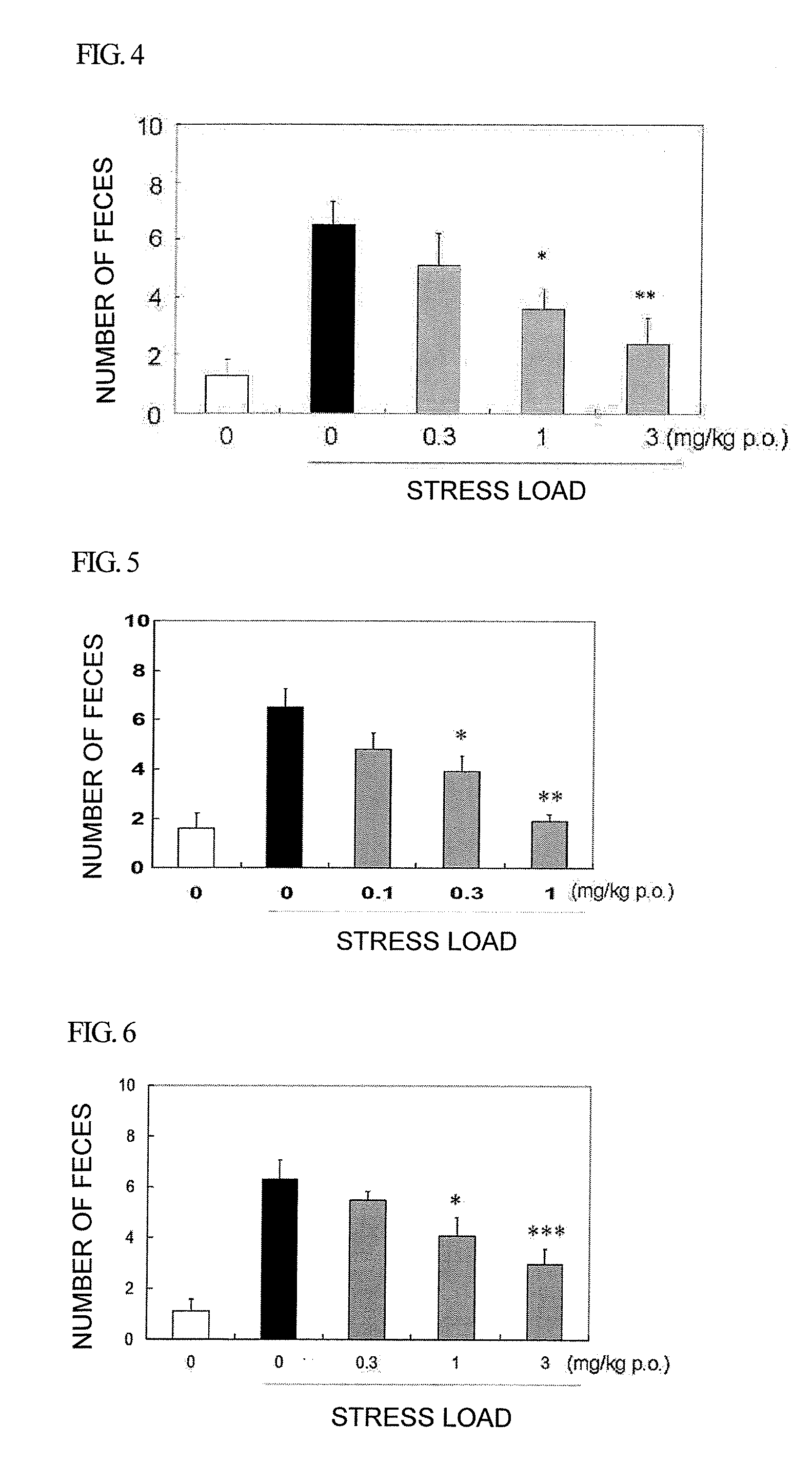
A compound of formula:wherein A, Ar, R3, R6, and m are disclosed herein, or a pharmaceutically acceptable salt thereof (a “Cyanoiminopiperazine Compound”), compositions comprising an effective amount of a Cyanoiminopiperazine Compound, and methods for treating or preventing pain, urinary incontinence, an ulcer, inflammatory-bowel disease, irritable-bowel syndrome, an addictive disorder, Parkinson's disease, parkinsonism, anxiety, epilepsy, stroke, a seizure, a pruritic condition, psychosis, a cognitive disorder, a memory deficit, restricted brain function, Huntington's chorea, amyotrophic lateral sclerosis, dementia, retinopathy, a muscle spasm, a migraine, vomiting, dyskinesia or depression in an animal comprising administering to an animal in need thereof an effective amount of a Cyanoiminopiperazine Compound are disclosed.
Owner:PURDUE PHARMA LP
Amide derivative or salt thereof
InactiveUS20090062363A1High antagonistic activitySuperior IBS-treating effectBiocideOrganic chemistryDisease5-HT6 receptor
[Problem] To provide a compound which can be used for the prevention and / or treatment of diseases in which 5-HT2B receptor and 5-HT7 receptor are concerned, particularly for the treatment of irritable bowel syndrome (IBS).[Means for Resolution] It was found that an amide derivative characterized by the possession of a nitrogen-containing bicyclic hetero ring (e.g., an indole or the like), or a pharmaceutically acceptable salt thereof, has a strong antagonism for both of the 5-HT2B receptor and 5-HT7 receptor. In addition, the compound of the present invention having the antagonistic activity for both of the receptors showed a good pharmacological action in comparison with the case in which an antagonist selective for either one of the receptors was used alone. Based on the above, the compound of the present invention is useful for the prevention and / or treatment of diseases in which 5-HT2B receptor and 5-HT7 receptor are concerned, particularly for the treatment of irritable bowel syndrome (IBS).
Owner:ASTELLAS PHARMA INC
Herbal composition for treating various disorders including psoriasis, a process for preparation thereof and method for treatment of such disorders
InactiveUS20030194456A1Safe and well-toleratedMinimal effectBiocideAntipyreticPhosphodiesteraseEnzyme inhibition
The invention provides a novel herbal composition containing the extracts of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant, optionally containing the extracts of the fruits of <italic>Cuminum cyminum< / highlight>, which exhibits useful in vitro, in vivo and interesting immunological and pharmacological activities; a process for preparation thereof; and a method of treatment of psoriasis and related immunological and biological disorders by administration of the said novel herbal composition. The useful in vitro, in vivo and interesting immunological and pharmacological activities exhibited by the extracts and fractions of the leaves and / or stem of <italic>Argemone mexicana < / highlight>plant include immunosuppression, lymphoproliferation inhibition, cytokine modulation such as IL-2 inhibition, IFNgamma inhibition, IL-10 induction, keratinocyte proliferation inhibition, keratolytic activity, endothelial cell proliferation inhibition, inhibition of cell adhesion molecule expression such as ICAM-1, MEST inhibition, and enzymes inhibition such as p60src Tyrosine kinase, which are known to be involved in anti-psoriatic activity. The novel herbal composition(s) is useful in the treatment of various disorders, such as psoriasis including plaque psoriasis, gutatte psoriasis, pustular psoriasis and psoriasis of the nails; dermatitis and scleroderma; eczema; inflammatory disorders and other autoimmune diseases like psoriatic arthritis, rheumatoid arthritis, Crohn's disease, multiple sclerosis, irritable bowel disease, ankylosing spondilitis, systemic lupus erythremetosus and Sjogren's syndrome; allergies like asthma and chronic obstructive pulmonary disease and is safe, well-tolerated, non-toxic, with minimal and reversible adverse reactions or side effects, and most importantly, with minimal relapse or recurrence of the disease following completion of a treatment regimen. The invention also describes the presence of phosphodiesterase (III, IV and V) inhibition and 5-Lipoxygenase inhibition in the aqueous, ethanolic or aqueous-ethanolic extracts of fruits of <italic>Cuminum cyminum < / highlight>plant.
Owner:LUPIN LTD
Use of methylcobalamin nasal spray to treat disorders
A method of treating a disorder by nasally administering methylcobalamin, with or without folinic acid. The disorders addressed are: a) attention deficit hyperactivity disorder (ADHD); b) anxiety, depression, stress and chronic stress; c) socialization problems, mood problems, behavior problems, memory problems; d) dislexia, depth perception problems, color viewing problems, visual and auditory processing problems, light modulation problems, night vision problems; e) speech problems such as finding words, apraxia, and articulation problems, sleep regulation problems, eye or muscle movement problems; and f) chronic fatigue problems, digestion problems, sensitivity to chemicals, viral infection, inflammatory conditions such as rheumatoid arthritis, sciatica, and fibromyalgia, asthma, irritable bowel, colitis, tinnitus, migraines, nail biting, autoimmune problems. In some embodiments, the disorders that are particularly addressed are ADHD, anxiety, stress and chronic stress, and irritable bowel.
Owner:KURTZ STAN
Magnetic Device
InactiveUS20080051621A1Assist in healing of woundUnique shapeElectrotherapyChiropractic devicesMenstruation problemsMagnetic effect
The present invention is directed to a magnetic device for treating conditions such as incontinence, upper and lower back pain, irritable bowel syndrome, arthritis in the hand and fingers, menstruation problems and prostate problems. The magnetic device in a preferred form consists of a mesh with spaced apart holding portions for magnets so that the device produces a synergistic magnetic effect to the desired area. The magnetic device in another preferred form consists of spaced apart magnets that are arranged about a desired area for treatment but maintaining the spacing relative to each other. In another preferred form, the magnetic device is a garment overlay which has spaced apart magnets on the overlay in positions that correspond to significant acupuncture points when the overlay is fitted. The garment overlay also has locating means for locating the position of the overlay relative to the desired body portion.
Owner:GT & MJ HLDG
Gastrointestinal transit disorder diagnosis composition and use thereof
InactiveCN102091335AReduce the number of inspectionsRelieve painEchographic/ultrasound-imaging preparationsX-ray constrast preparationsGastrointestinal transitGastrointestinal obstructions
The invention discloses a gastrointestinal transit disorder diagnosis composition. The composition which can disintegrate at fixed time and fixed position in the gastrointestinal tract consists of an inner body, an outer layer covering the inner body and a gastrointestinal transit disorder diagnosis probe material which is mixed in the inner body or / and the outer layer. According to the evaluation of information on the probe marker material-containing preparation, such as position in the gastrointestinal tract, retention and image characteristics after disintegration, after a receiver takes the composition orally, the composition can be used for the diagnosis, positioning and degree and property judgment of clinic gastrointestinal transit disorder, such as gastrointestinal obstruction caused by benign and malignant occupation, and the identification and diagnosis of gastrointestinal transit disorder, such as redundant colon and irritable bowel syndrome.
Owner:张发明
Probiotic composition useful for dietary augmentation and/or combating disease states and adverse physiological conditions
Owner:COBB & ASSOCS
5-HT3 receptor antagonists and methods of use
The subject invention provides useful and novel 5-HT3 antagonists. The subject invention also provides methods for synthesizing the compounds of invention. The invention also provides methods for the treatment of irritable bowel syndrome and other such conditions.
Owner:ARYX THERAPEUTICS
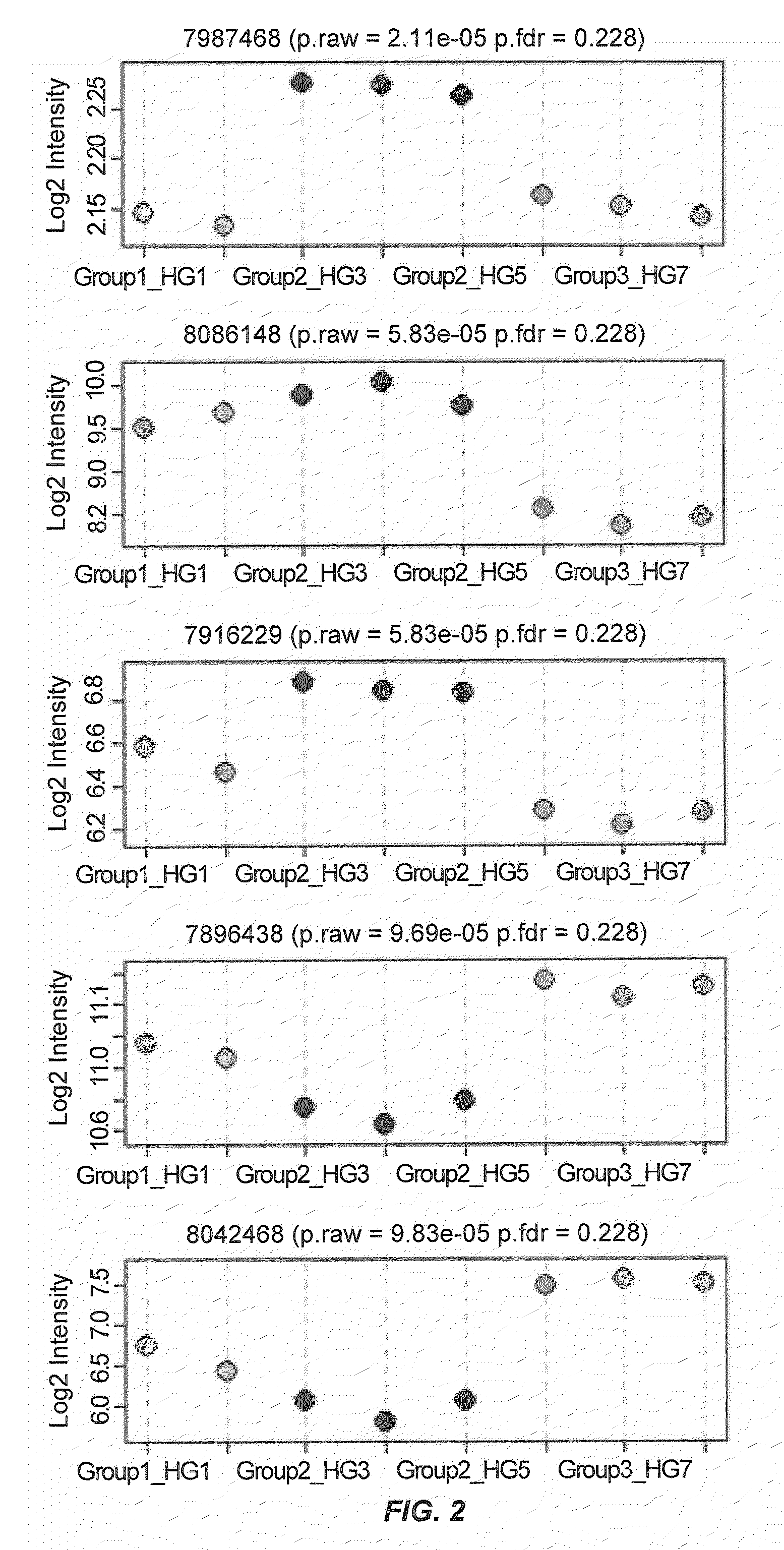
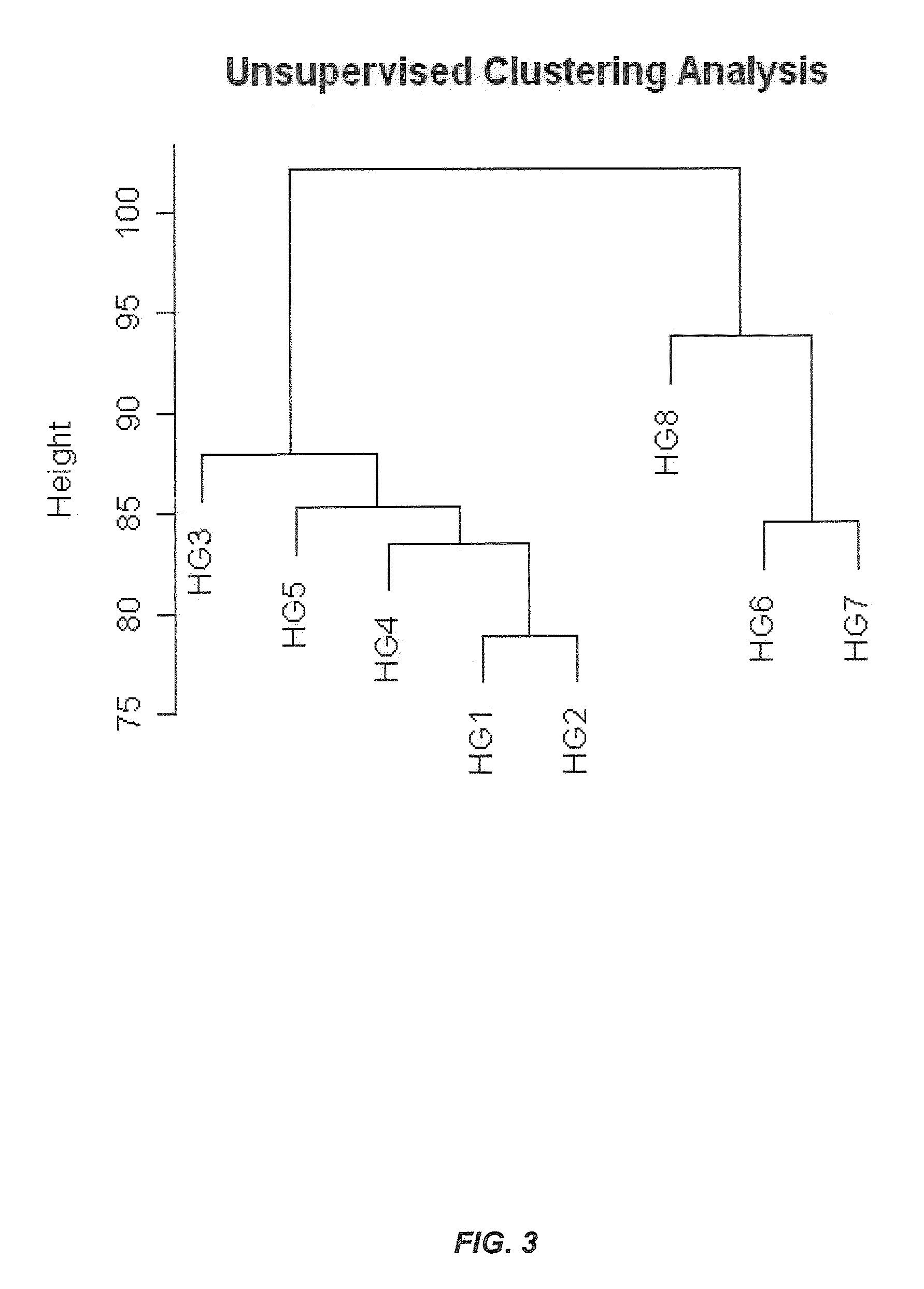
Novel genomic biomarkers for irritable bowel syndrome diagnosis
InactiveUS20130005596A1Accurate classificationAccurate predictionAntibacterial agentsNervous disorderGenomic BiomarkerGenomic biomarkers
The invention provides novel biomarkers, kits, and methods of diagnosing, prognosing, and subtyping IBS. In one aspect, the invention provides novel genomic biomarkers for diagnosing, classifying, providing a prognosis for, and assigning therapy for IBS in a subject in need thereof. In another aspect, the present invention provides novel algorithms for the diagnosis and prognosis of IBS.
Owner:NESTEC SA
Compound probiotics lactobacillus powder for treating irritable bowel syndrome (IBS) and application thereof
ActiveCN110169983AEffective treatmentSymptoms improvedPowder deliveryOrganic active ingredientsGut floraNutrients substances
The application provides compound probiotics powder for treating irritable bowel syndrome (IBS). The compound probiotics powder is prepared from lactobacillus casei Zhang, bifidobacterium animalis subsp. lactis V9 and lactobacillus plantarum P-8; the compound probiotics powder is obtained through optimizing, compounding and combining nutrient substances with different properties; the compound probiotics powder can regulate intestinal flora, effectively treats the IBS, can obviously improve symptoms, and reduces inflammatory responses, thereby solving the problems that existing compound probiotics lactobacillus powder is single in flora composition, low in viable count, and poor in probiotics safety, and is lacking in probiotics preparations which effectively treat the IBS; the compound probiotics powder has wide market prospects.
Owner:BEIJING SCITOP BIO TECH CO LTD
Therapeutic agent for irritable bowel syndrome
ActiveUS20090093415A1Excellent IBS-treating effectImprove efficacyBiocidePeptide/protein ingredientsIntestinal structureNK1 receptor antagonist
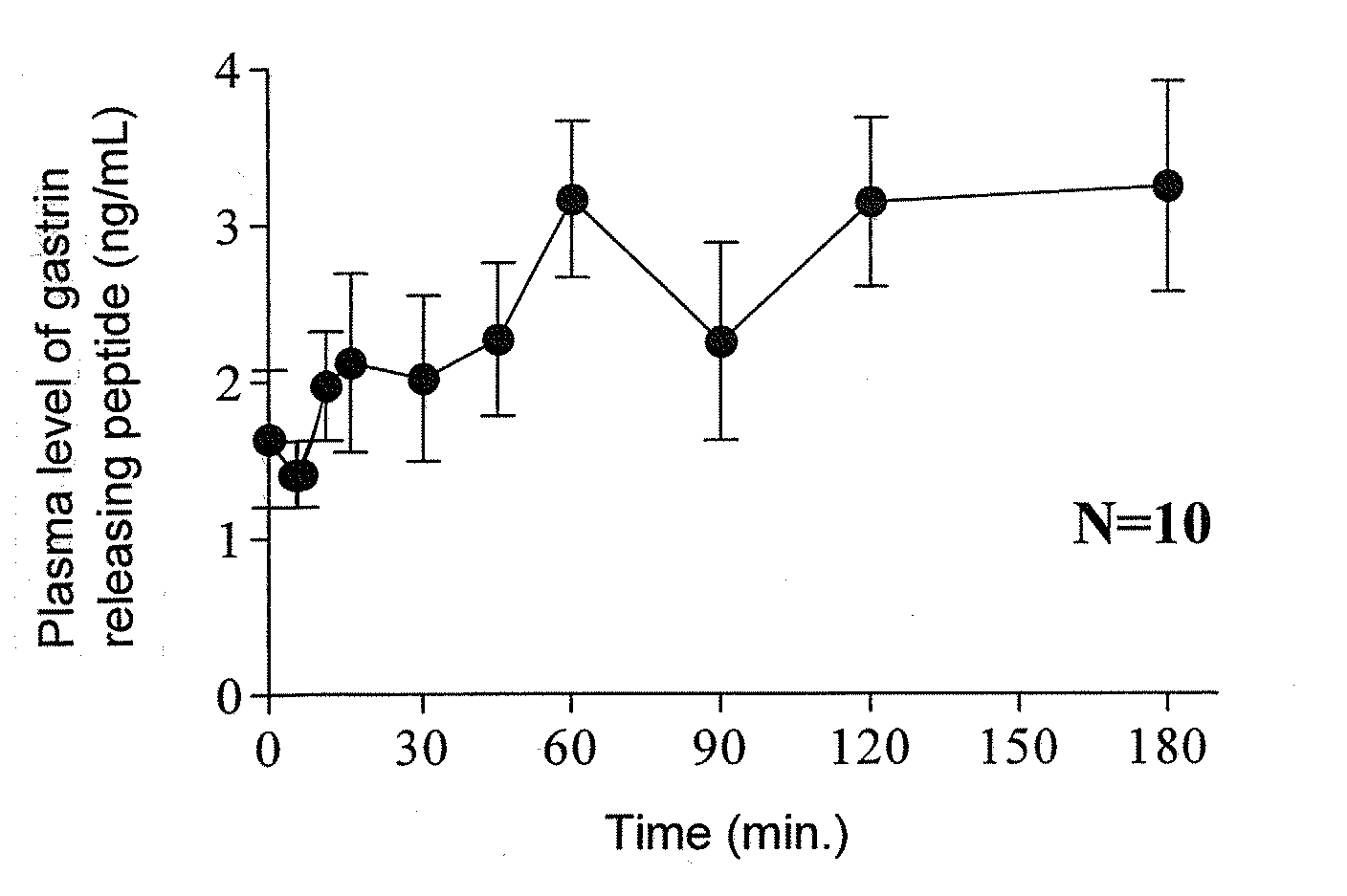
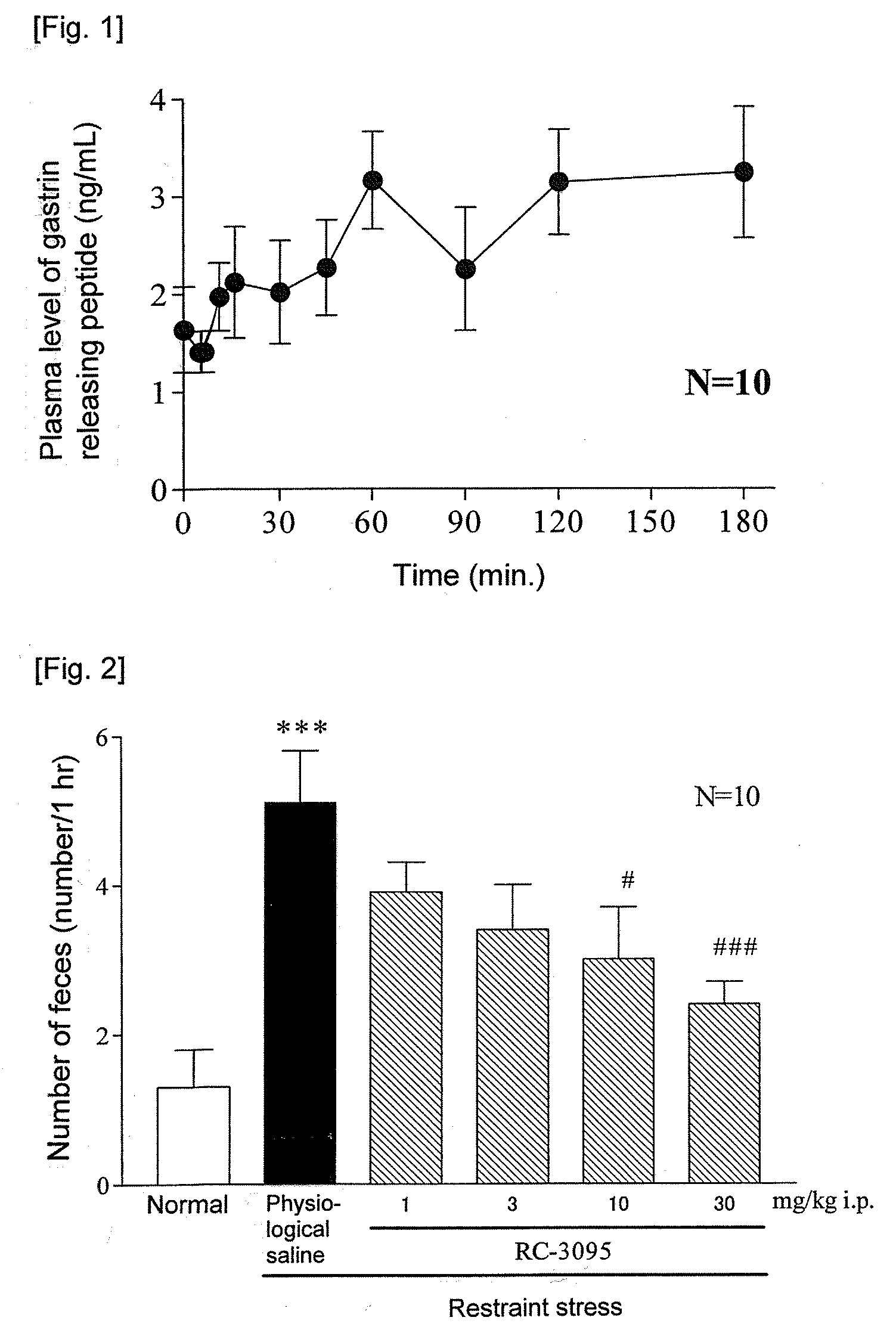
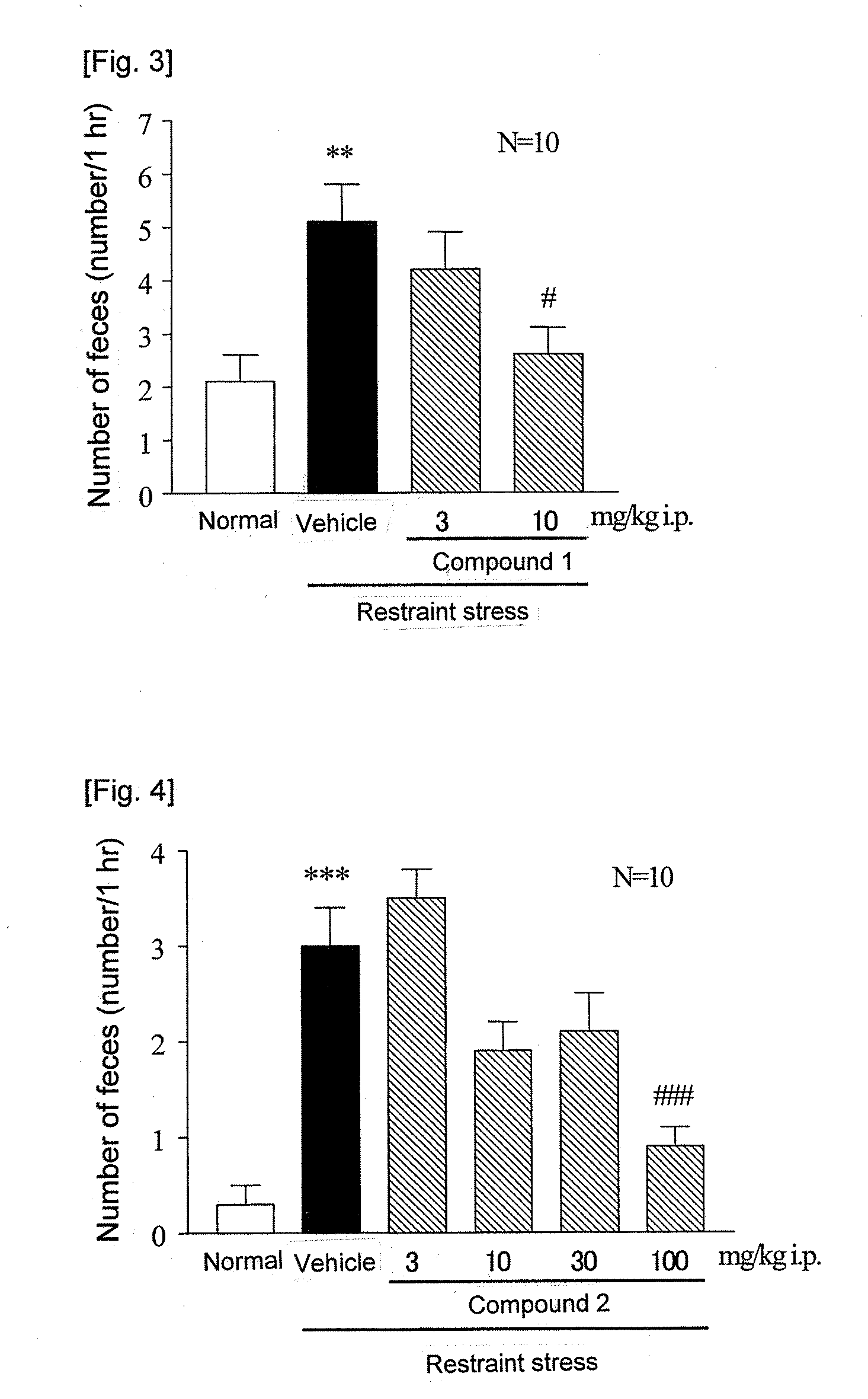
[Problem]To provide a therapeutic agent for irritable bowel syndrome (IBS), which is excellent in efficacy and safety.[Means for Resolution]It was shown that the bombesin 2 (BB2) receptor antagonists typified by RC-3095 are therapeutic agents for irritable bowel syndrome (IBS), which show excellent efficacy in both of an abdominal symptom and bowel movement disorder. Thus, according to the present invention, it became possible to provide a therapeutic agent for irritable bowel syndrome (IBS) which comprises, as an active ingredient, a bombesin 2 (BB2) receptor antagonist exerting an excellent efficacy in both an abdominal symptom and bowel movement disorder.
Owner:SELDAR PHARMA
Method for Treatment of Constipation-Predominant Irritable Bowel Syndrome
ActiveUS20080031983A1Effective treatmentIncrease the number ofBiocideDigestive systemProanthocyanidinConstipation predominant irritable bowel syndrome
The present invention provides methods for treating constipation-predominant irritable bowel syndrome comprising administering to a patient in need thereof, a polymeric proanthocyanidin composition from a Croton species or Calophyllum species in an amount sufficient to treat constipation-predominant irritable bowel syndrome (c-IBS). Treatment of c-IBS includes the treatment of the constipation component of c-IBS as well as the pain and abdominal discomfort associated with c-IBS. In one embodiment, the polymeric proanthocyanidin compound is crofelemer. The present invention in an alternative embodiment also provides methods for treating alternating constipation-predominant / diarrhea-predominant irritable bowel syndrome.
Owner:NAPO PHARMA INC
Treatments for Gastrointestinal Disorders
The present invention features peptides, compositions, and related methods for treating gastrointestinal disorders and conditions, including but not limited to, irritable bowel syndrome (IBS), gastrointestinal motility disorders, functional gastrointestinal disorders, gastroesophageal reflux disease (GERD), duodenogastric reflux, Crohn's disease, ulcerative colitis, inflammatory bowel disease, functional heartburn, dyspepsia, visceral pain, gastroparesis, chronic intestinal pseudo-obstruction (or colonic pseudo-obstruction), disorders and conditions associated with constipation, and other conditions and disorders are described herein, using peptides and other agents that activate the guanylate cyclase C (GC-C) receptor.
Owner:IRONWOOD PHARMA
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS9907755B2Antibacterial agentsMetabolism disorderAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Methods for treating apnea, apnea disorders, bulimia, and other disorders using optically pure (+) norcisapride
InactiveUS6048879ARelieve symptomsReducing and avoiding adverse effectBiocideNervous disorderDiseaseNorcisapride
Methods for the prevention, treatment or management of apnea, apnea disorders, bulimia nervosa, irritable bowel syndrome, urinary incontinence, bradycardia, bradyarrhythmia, syncope, other disorders, or symptoms thereof using (+) norcisapride, or a pharmaceutically acceptable salt thereof, substantially free of its (-) stereoisomer.
Owner:SEPACOR INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com