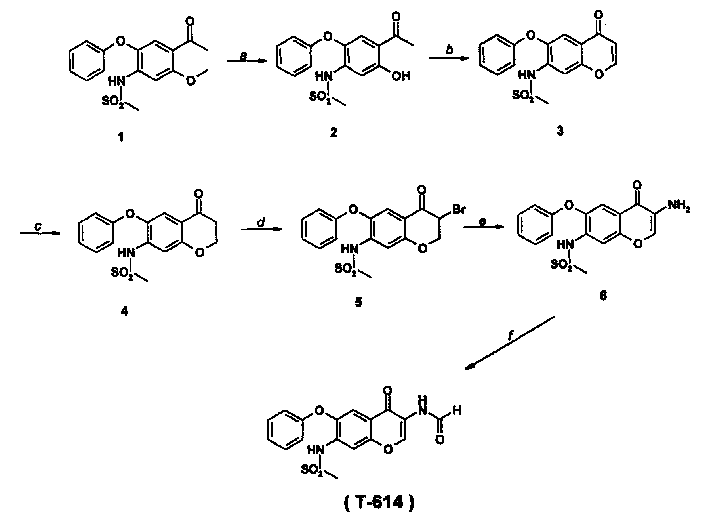
Preparation of 3-(formamide)-7-(methylsulfonyl amine)-6-(phenoxy)-4H-1-(benzopyran)-4-ketone
A technology of methoxyacetophenone and methanesulfonamide, which is applied in the field of preparing anti-inflammatory drug 3--7--6--4H-1-benzopyran-4-one, can solve the complicated after-treatment, Problems such as low reaction yield and high toxicity are not suitable for large-scale synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Preparation of 2-acetyl-4-phenoxy-5-methanesulfonamidophenol (compound 2)
[0014] 54L acetonitrile, 3.0kg anhydrous AlCl 3 Add 3.7kg of compound 1 into the reaction kettle, add 1.9kg of sodium iodide at 0-5°C, react at room temperature for 3 hours, after the reaction is complete, add 1% sodium thiosulfate under ice water cooling, filter, add water to the filtrate to precipitate a solid , filtered, and vacuum-dried to obtain 2-acetyl-4-phenoxy-5-methanesulfonamide phenol (compound 2), yield: 86%.
[0015] 1 H NMR (300MHz, CDCl 3 )δ: 2.45 (3H, s), 3.10 (3H, s), 6.94-7.40 (m, 10), 12.44 (1H, s);
[0016] EI-Ms (m / z, int.%): 321 (M + , 100), 242(21.54), 306(20.60), 199(18.80), 322(18.34), 144(16.65), 200(16.33), 43(15.33).IR(υ KBr max ): 3254, 1634, 1600, 1569, 1504, 1493, 1430, 1409cm -1 .
Embodiment 2
[0017] Example 2: Preparation of 7-(methylsulfonamide)-6-(phenoxy)-4H-1-benzopyran-4-one (compound 3)
[0018] Suspend compound 23.05Kg in triethyl orthoformate, add 2.5L perchloric acid at room temperature, react for 1-24h, after the reaction is complete, filter, boil in water for 1-3h, and drain to obtain compound 7-(methanesulfonamide)- 6-phenoxy-4H-1-benzofuran-4-one (compound 3), yield: 85%.
[0019] 1 H NMR (300MHz, CDCl 3 )δ: 3.18(3H, s), 6.28(1H, d), 7.05(2H, d), 7.26-7.27(2H, m), 7.44(2H, m), 7.57(1H, s), 7.77(1H ,s), 7.83(1H,d).
[0020] EI-Ms (m / z, int.%): 3 31 (M + , 100), 251(95.56), 332(20.10), 252(18.23), 168(16.15), 154(15.28), 149(15.07), 196(13.66).IR(υ KBr max ): 3027, 1625, 1594, 1567, 1473, 1444, 1325, 1305cm -1 .
Embodiment 3
[0021] Example 3: Preparation of 7-(methylsulfonamide)-6-(phenoxy)-4H-1-(benzo-2,3-dihydrofuran)-4-one (compound 4)
[0022] Add the mixed solution of 2.72kg compound 3, 30L tetrahydrofuran and methanol into the reaction kettle, stir to make compound 3 dissolve completely, and 2 Under protection, 0.79 kg of palladium carbon was fully wetted with ethanol and added to the reaction kettle, and then 13.44 kg of ammonium formate was added in batches at room temperature, reacted for 2-3 hours, filtered, concentrated, and dried to obtain compound 4, yield: 90 %.
[0023] 1 H NMR (300MHz, CDCl 3 )δ: 2.75 (2H, t), 3.13 (3H, s), 4.52 (2H, t), 6.97-7.37 (m, 7H);
[0024] EI-Ms (m / z, int.%): 333 (M + , 100), 254 (72.42), 198 (43.58), 144 (23.82), 170 (21.56), 77 (19.16), 334 (16.6).
[0025] IR(υ KBr max): 3254, 1674, 1618, 1578, 1497, 1450, 1394, 1322, 1270, 1219, 1166, 1140.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com