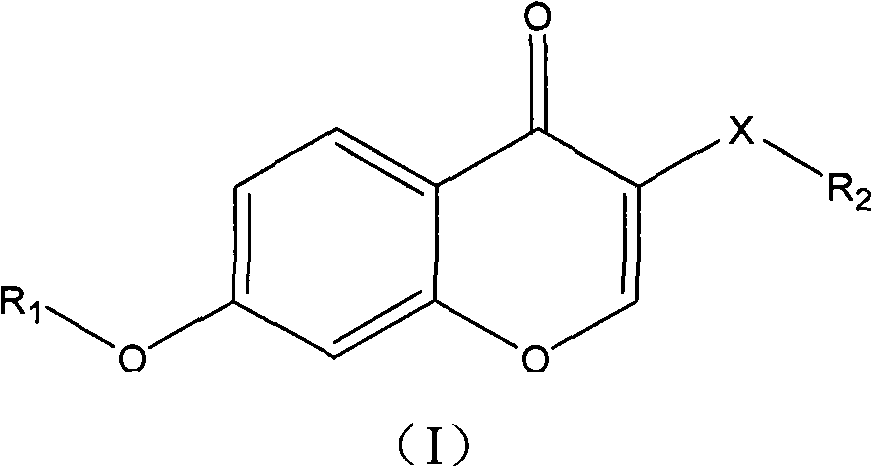
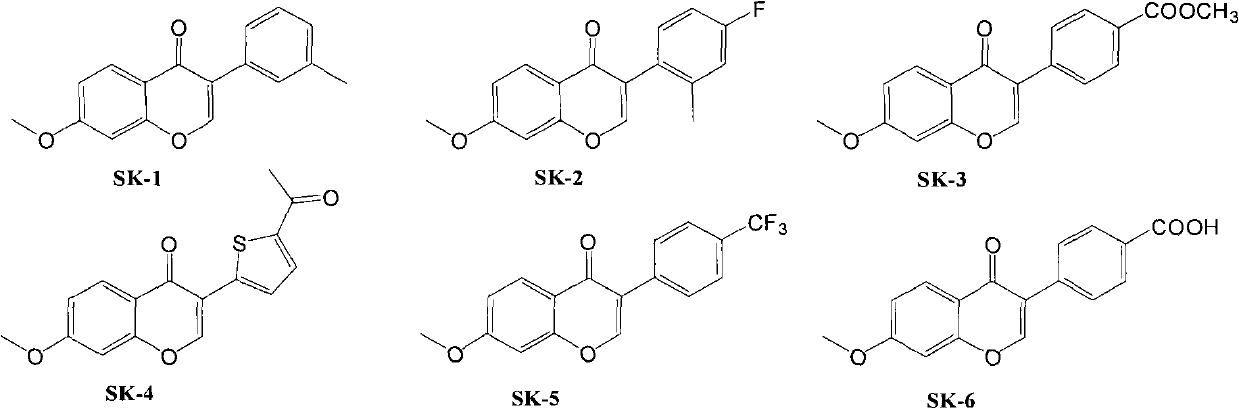
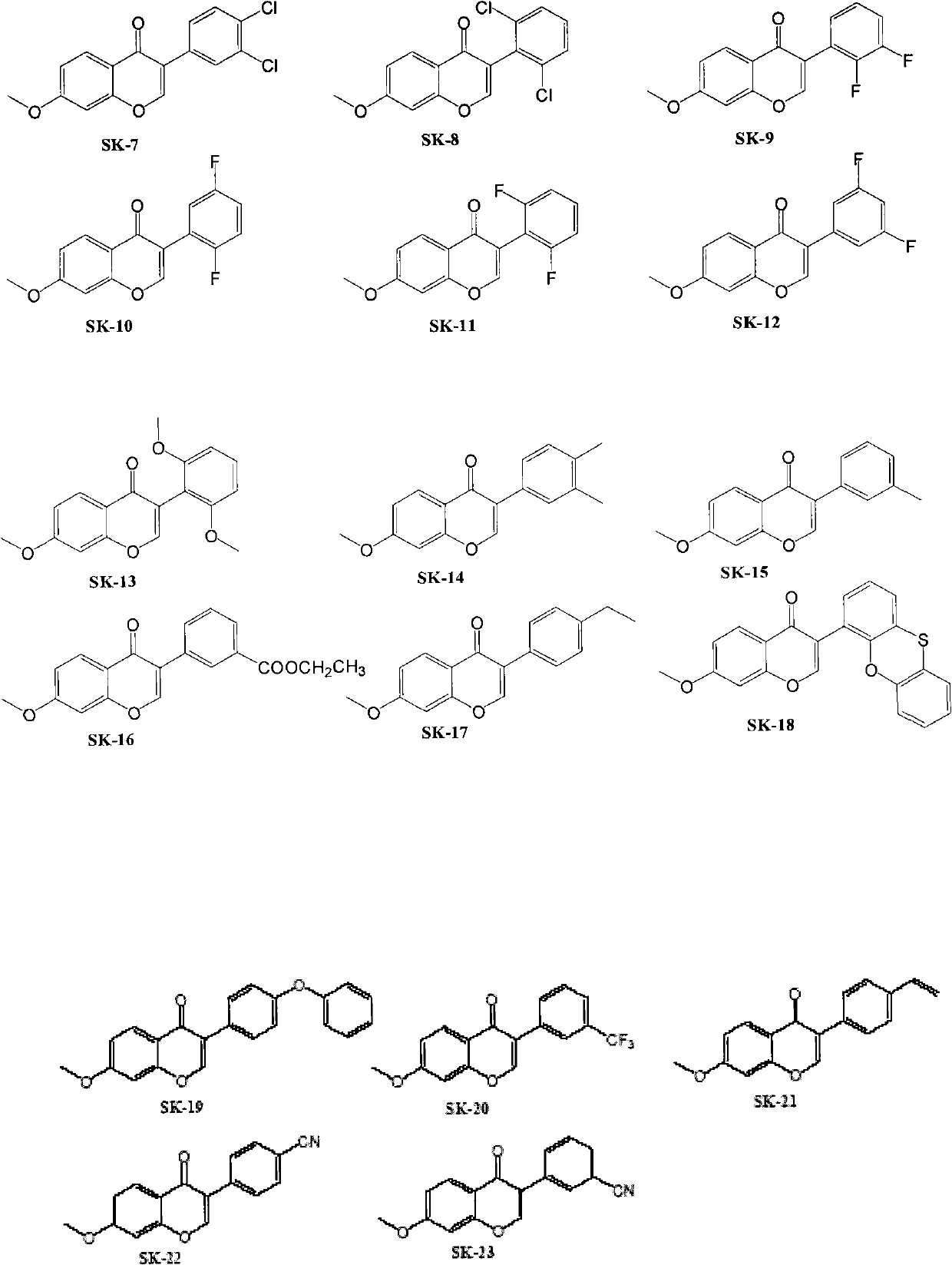
Isoflavone compound, its preparation method, and its application in preparation of antiviral or antitumor drugs
A compound and selected technology can be used in anti-tumor drugs, anti-viral agents, active ingredients of heterocyclic compounds, etc., and can solve problems such as increasing difficulty, increasing investment in research and development, and prolonging the time for research and development of new drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of Compound 2 in Scheme 1
[0041] Sodium block (12.7g, 550mmol) was put into dried xylene (100mL), heated to melt, cooled to room temperature, poured out xylene, washed with anhydrous ether (50mL×2). Sodium was placed in anhydrous ether (100 mL), stirred vigorously, and cooled to 0°C. Under nitrogen protection, an anhydrous ether solution (100 mL) of paeonol 1 (30.7 g, 185 mmol) and ethyl formate (41.0 g, 550 mmol) was slowly added dropwise to the mixture. After the addition was complete, stirring was continued at 0°C for 1 hour, then warmed to room temperature overnight. The reaction solution was poured into ice water (400 mL) containing 12.5% acetic acid, extracted with ethyl acetate (200 mL×3), the organic phases were combined, and the solvent was evaporated to dryness under reduced pressure to obtain 33.0 g of a light yellow solid with a yield of 92%. It was confirmed that the obtained compound was compound 2 in Scheme 1.
[0042] 1 ...
Embodiment 2
[0043] Embodiment 2: the preparation of compound 3
[0044] Mix 3-(2-hydroxy-4-methoxyphenyl)-3-carbonylpropionaldehyde 2 (33g, 170mmol) obtained in Example 1 with acetic acid (150mL) and concentrated hydrochloric acid (10mL), Heat for half an hour. The acetic acid was distilled off under reduced pressure, water (300 mL) was added and adjusted to pH=8 with sodium bicarbonate. Extracted with dichloromethane (200 mL×3), combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 28.0 g of a pale yellow solid with a yield of 93%. It was confirmed that the obtained compound was compound 3 in Scheme 1.
[0045] 1 H NMR (300MHz, CDCl 3 ): 3.90 (s, 3H, CH 3 ), 6.28 (d, 1H, J=6.3Hz, O=C-CH=C), 6.84 (d, 1H, J=2.4Hz, ArH), 6.98 (dd, 1H, J=2.4Hz, 9.0Hz, ArH), 7.78 (d, 1H, J=6.3Hz, C=CH-O), 8.12(d, 1H, J=9.0Hz, ArH); ESI-MS m / z: 177 (M+H) + .
Embodiment 3
[0046] Embodiment 3: the preparation of compound 4
[0047] The 7-methoxybenzopyrone 3 (4.3g, 24.4mmol) and piperidine (6.2mL, 62.5mmol) obtained in Example 2 were dissolved in methanol (50mL), refluxed for 3 hours, and evaporated to dryness under reduced pressure Solvent was used to obtain 6.3 g of yellow solid with a yield of 99%. It was confirmed that the obtained compound was compound 4 in scheme 1.
[0048] 1 H NMR (300MHz, CDCl 3 ): 1.68 (m, 6H, 3CH 2 ), 3.39 (m, 4H, 2CH 2 ), 3.81 (s, 3H, CH 3 ), 5.78 (d, 1H, J=12.3Hz, O=C-CH=C), 6.37 (dd, 1H, J=2.4Hz, 9.0Hz, ArH), 6.41 (d, 1H, J=2.4Hz, ArH), 7.58(d, 1H, J=9.0Hz, ArH), 7.81(d, 1H, J=12.3Hz, C=CH-N), 14.5(s, 1H, OH); ESI-MS m / z : 262(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com