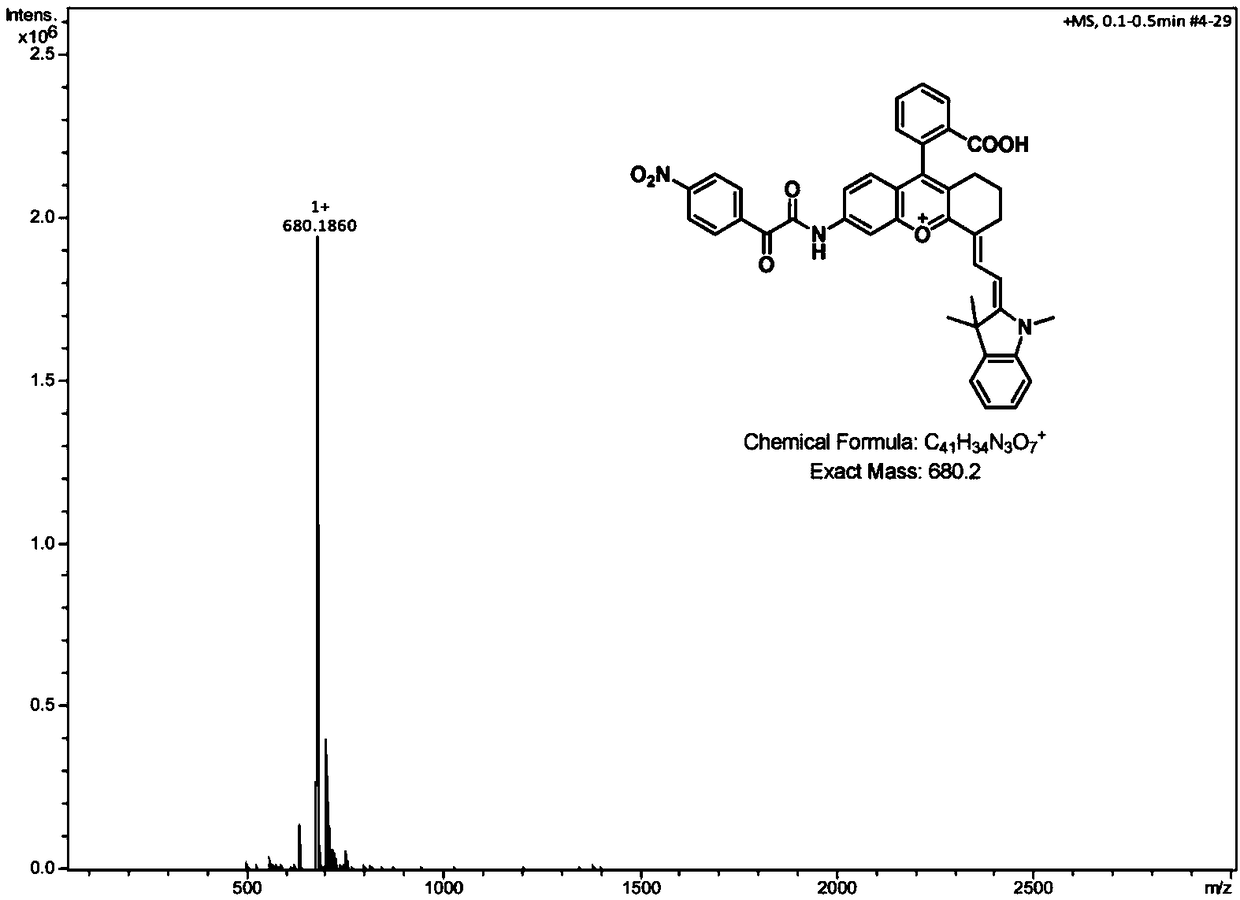
Amido benzopyran cyanine fluorochrome and probe as well as synthetic method and application thereof
A technology of pyranocyanine and fluorescent dyes, which is applied in the fields of chemistry and biology, can solve the problems of difficult modification of cyanine dyes, poor stability, and influence on promotion, and achieve good light stability, reduce light damage, and enhance penetration ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 intermediate 5
[0060] The synthetic route of intermediate 5 is as follows, specifically comprises the following steps:
[0061]
[0062] Put 46mmol of compound 1 and 4.4mL of acetic anhydride in a round-bottomed flask, add 200mL of tetrahydrofuran to dissolve, then stir the reaction at room temperature for 2h, remove the tetrahydrofuran in the reaction solution after the reaction is complete, and obtain compound 2 after recrystallization;
[0063] Take 4mmol of compound 2, 4mmol of phthalic anhydride and 10mmol of aluminum trichloride in a round-bottomed flask, add 13mL of 1,1,2,2-tetrachloroethane under ice cooling until the reactants are completely dissolved, and react at room temperature for 2h. Then the reaction was heated to 100°C and stirred for 12 hours; after the reaction was completed, it was cooled to room temperature, poured into ice water to precipitate a solid, and compound 3 was obtained;
[0064] Take 1 mmol of compou...
Embodiment 2
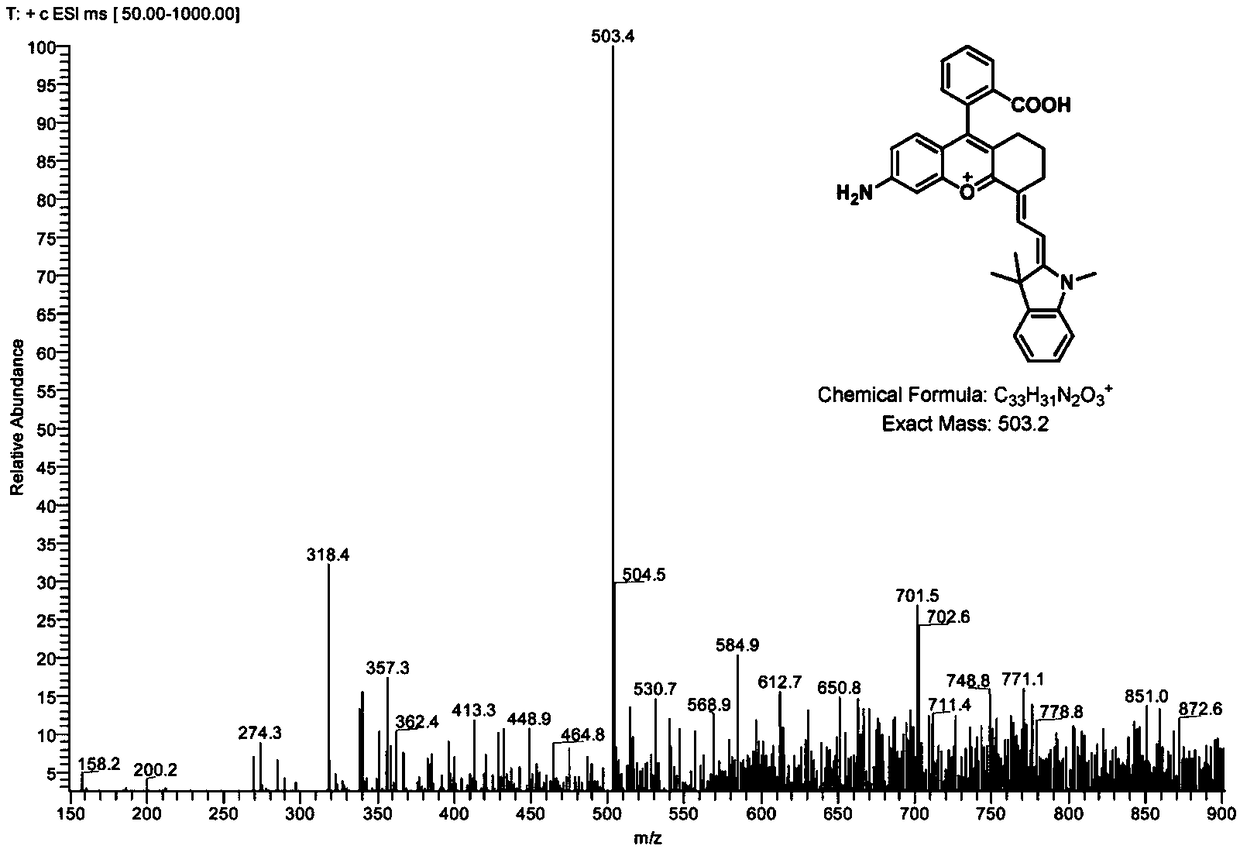
[0066] Embodiment 2 Aminobenzopyranocyanine fluorescent dye NIR-NH of type I structural formula 2 Synthesis
[0067] 0.2mmol of intermediate 5 (prepared in Example 1) and 0.2mmol of X are C(CH 3 ) 2 , R 1 is methyl, L is hydrogen, and m is 0, compound 6-1 (structural formula is as follows) is placed in a round-bottomed flask, mixed in 4 mL of acetic anhydride solution, and stirred at room temperature for 1.5 h; The instrument was spin-dried under reduced pressure, and purified with a silica gel column to obtain intermediate 8-1 (structural formula is as follows).
[0068]
[0069] Intermediate 8-1 (0.11g, 0.2mmol) was dissolved in 6mL of acetic acid / concentrated hydrochloric acid (1:2, v / v) solution, stirred at 110°C for 2h, then cooled to room temperature, and the mixture was poured into ice water , basified with sodium hydroxide solution, stirred at room temperature for 0.5h, then extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, t...
Embodiment 3
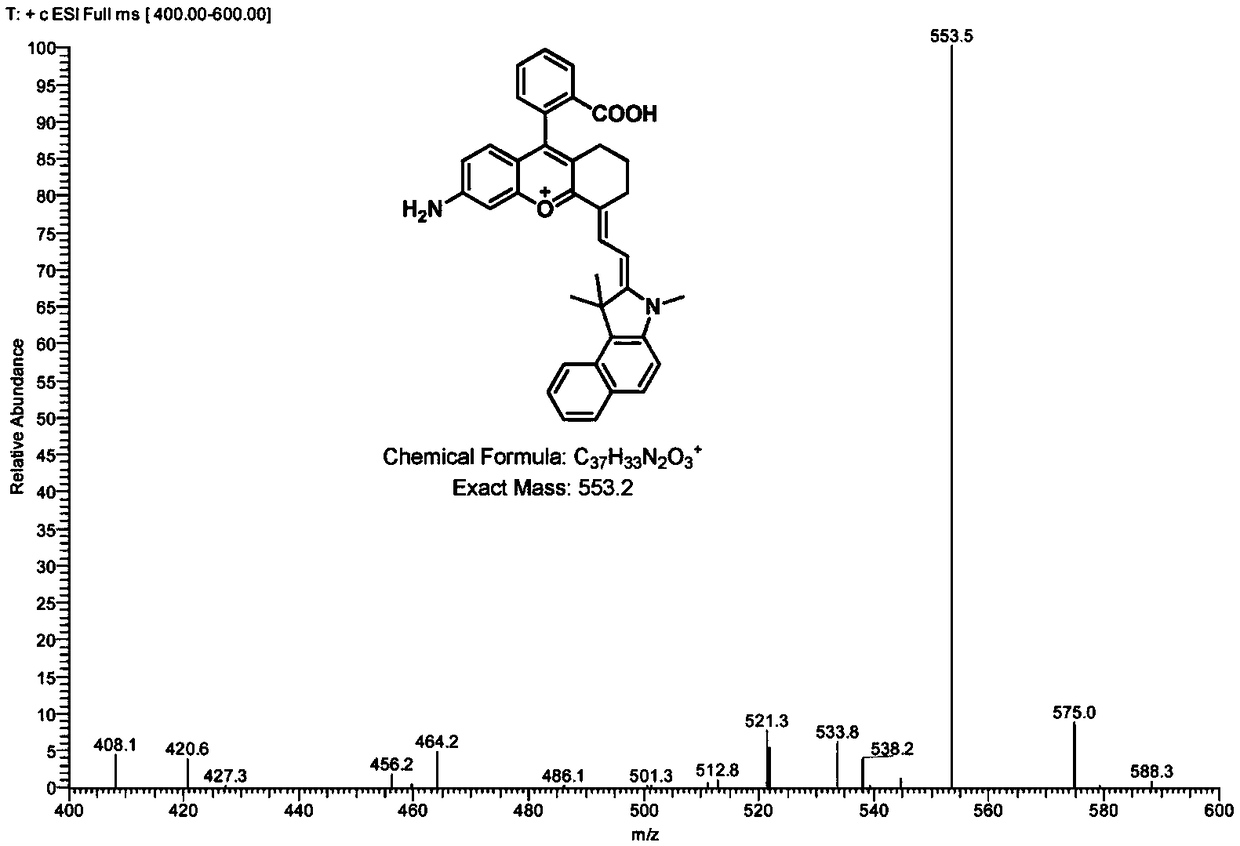
[0073] Example 3 Aminobenzopyranocyanine Fluorescent Dye Ben-NIR-NH of Type II Structural Formula 2 Synthesis
[0074] 0.2mmol of intermediate 5 (prepared in Example 1) and 0.2mmol of X are C(CH 3 ) 2 , R 1 is methyl, L is hydrogen, and m is 0, compound 7-1 (structural formula is as follows) is placed in a round-bottomed flask, mixed in 4 mL of acetic anhydride solution, and stirred at room temperature for 2 hours; after the reaction is complete, the reaction solution is It was spin-dried under reduced pressure and purified by a silica gel column to obtain intermediate 9-1 (structural formula is as follows).
[0075]
[0076] Intermediate 9-1 (0.138g, 0.2mmol) was dissolved in 6mL of acetic acid / concentrated hydrochloric acid (1:2, v / v) solution, stirred at 110°C for 2h. Then it was cooled to room temperature, the mixture was poured into ice water, basified with sodium hydroxide solution, stirred at room temperature for 0.5h, then extracted with dichloromethane, the org...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com