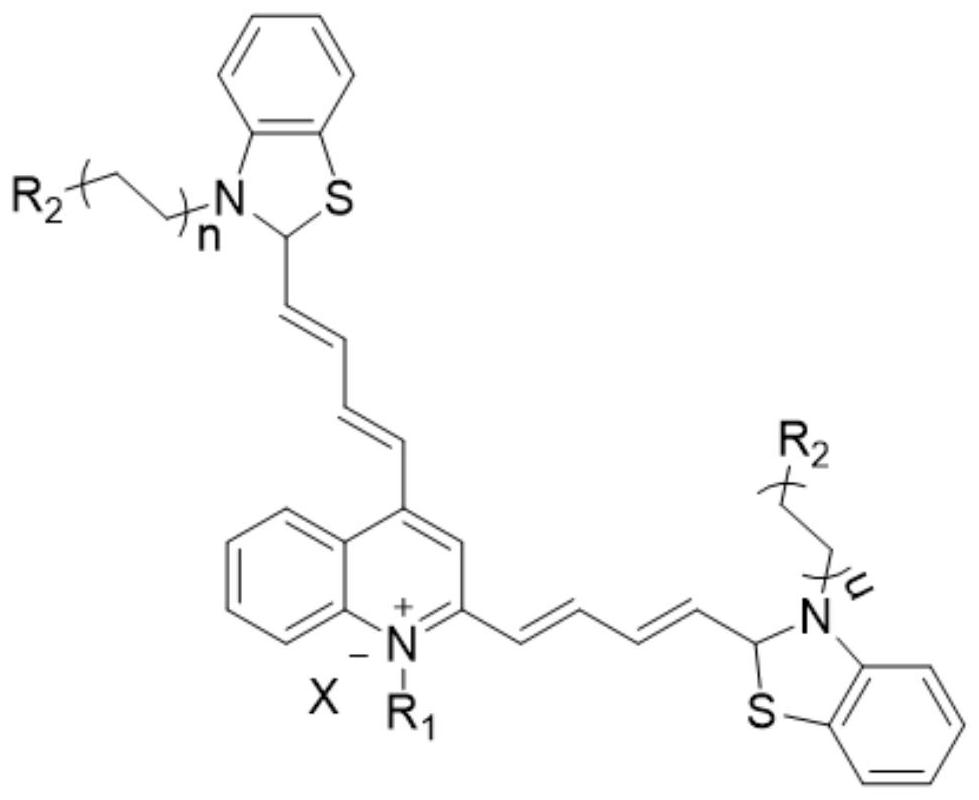
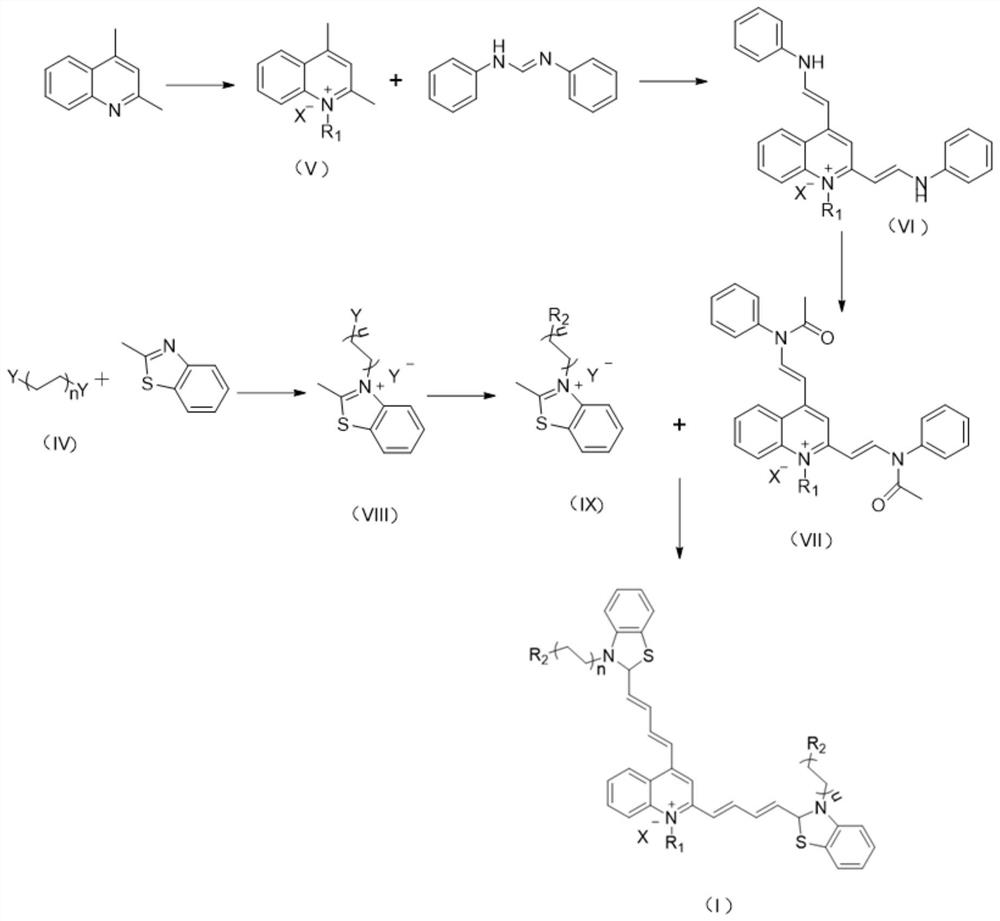
Thiazole orange derivative, preparation and application thereof
A technology of orange derivatives and thiazoles, applied in the field of small molecule photothermal materials, can solve the problem of low photothermal conversion efficiency of mitochondrial photothermal probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The embodiment of the present application provides a thiazole orange derivative (I-b), the synthesis method of which comprises:
[0082] (1) Add 2,4-dimethylquinoline and methyl iodide to acetonitrile solvent at a molar ratio of 1:1, react at 60°C for 24 hours, cool to room temperature, add an appropriate amount of ethyl acetate dropwise, and solids precipitate out. Collect solid, purify, obtain product formula (Ⅴ-b);
[0083]
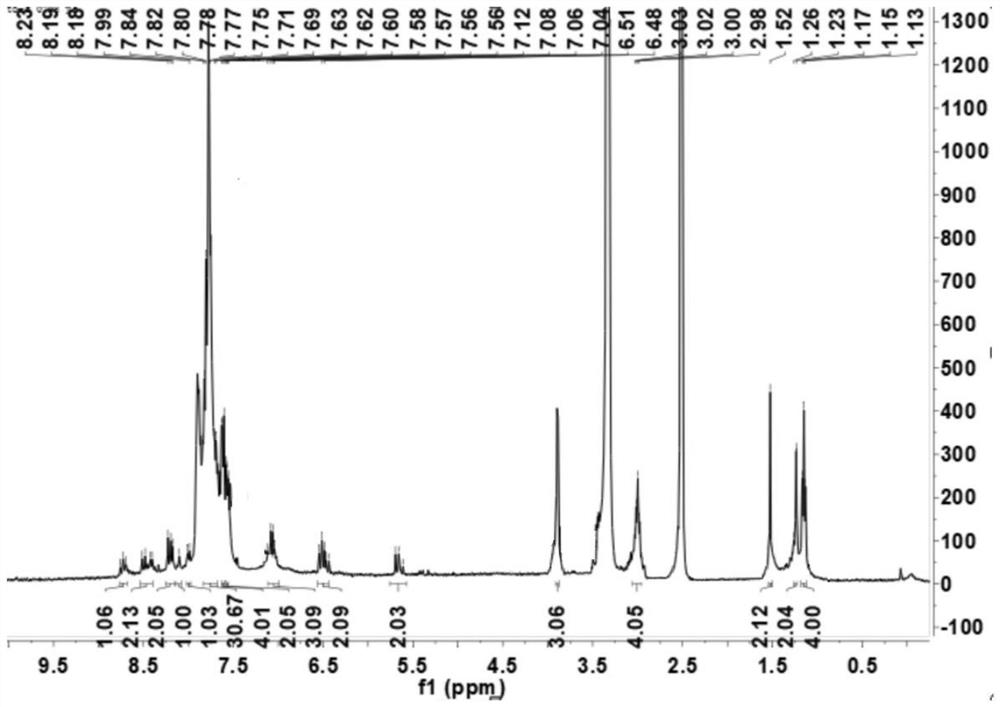
[0084] The NMR data of formula (Ⅴ-b) is: 1 H NMR (DMSO, 400Hz): δ8.13 (d, J = 8.4Hz, 1H), 8.04 (d, J = 8.4Hz, 1H), 7.68 (m, 2H), 6.92 (s, 1H), 4.33 ( s,3H), 2.68(s,3H), 2.59(s,3H); ESI-MS m / z299.02[M-I] + ;
[0085] (2) Dissolve the formula (V-b) and N,N'-diphenylformamidine in ethanol at a molar ratio of 1:2, and heat at reflux at 60°C for 1 hour. After cooling to room temperature, the precipitate was collected by filtration, washed three times with ethanol, and then dried in vacuo to obtain a brown solid product formula (VI-b);
[0086...
Embodiment 2
[0102] The embodiment of the present application provides the photothermal conversion performance test of the compound of the formula I-b structure in Example 1, including:
[0103] The formula (I-b) prepared in Example 1 and commercialized ICG (indocyanine green ICG is a kind of cyanine dye used in medical diagnosis) are diluted to 1mM, 2mM, 3mM, 4mM, 5mM with deionized water Concentration of five gradients, then irradiated with 808nm laser for 5 minutes, irradiation energy is 2J / cm 2 , detect the ambient temperature every 1 minute with a near-infrared temperature detector, and the control group uses deionized water to measure the formula (I-b) and commercial ICG prepared in Example 1 when the irradiation energy is 2J / cm 2 The temperature rise within 5 minutes under the irradiation of the 808nm laser, the results are as follows Figure 4 ~ Figure 5 as shown, Figure 4 The formula (I-b) and ICG provided for the embodiment of the application are 2J / cm at the irradiation energ...
Embodiment 3
[0105] The embodiment of the present application provides the cytotoxicity test of the compound of formula I-b structure in embodiment 1, including:
[0106] Cell culture: Inoculate tumor cells (human prostate cancer PC3, breast cancer MCF7, non-small cell lung cancer A549 and liver cancer HepG2 cell lines) and Hacat epidermal cells in cell culture flasks and place at 37°C, 5% CO 2 The medium is cultured in the environment, and the medium is 1640 medium containing 10% fetal bovine serum and 0.5% double antibody. Cell inoculation: Inoculate the cultured cells in a 96-well plate with a cell density of 8000 cells / mL, and continue to store at 37°C, 5% CO 2 Incubate in the environment for 48 hours. Add gradient compound solution: remove the medium in the 96-well plate, wash with pre-cooled PBS 3 times, add 1640 complete medium containing formula (I-b) of different gradient Example 1, and continue to store at 37°C, 5% CO 2 Incubate in the environment for 24 hours. Add MTT solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com