Long wavelength emitting fluorescence probe for specifically detecting cysteine in living cells and preparation method and application thereof
A fluorescent probe, cysteine technology, applied in the field of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
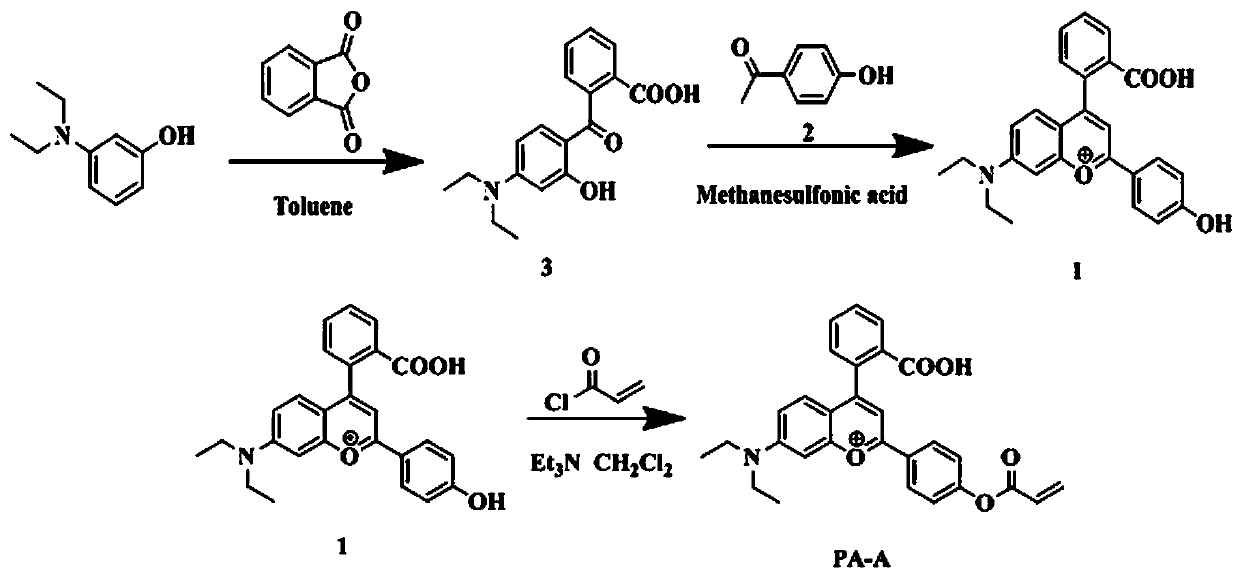
[0033] Example 1: Preparation of compound 1: N-diethylaminophenol (1.8 g, 10.9 mmol) and phthalic anhydride (1.8 g, 12.2 mmol) were reacted in 200 mL of benzene at 130 °C for 12 hours to obtain White solid, then white solid (0.626 g, 2 mmol) was added to 10 mL of methanesulfonic acid containing p-hydroxyacetophenone (0.272 g, 2 mmol), reacted at 90 ° C for 8 hours, cooled and added to 200 mL of ice Water, add 10 mL perchloric acid, extract with dichloromethane (50 mL × 3), dry and rotary evaporate to dryness, use dichloromethane and glacial acetic acid mixed solvent (volume ratio: 10:1) to separate by column chromatography Compound 1 was obtained with a yield of 33%. Synthetic route such as figure 1 shown.
Embodiment 2
[0034] Example 2: Preparation of fluorescent probes for detecting biothiols in water-soluble environments according to the present invention: Compound 1 (600 mg, 1.45 mmol) was mixed with acryloyl chloride (468 μL, 5.8 mmol), triethylamine (804 μL, 5.8 mmol) in 30 mL redistilled dichloromethane, stirred in an ice bath for 1 hour, then returned to room temperature and stirred for 16 hours. Diluted with 30 mL of dichloromethane, washed with water (50 mL × 3), dried and spun to dryness, and separated by column chromatography with a mixed solvent of dichloromethane and methanol (volume ratio 30:1) to obtain the target compound PA-A , Yield: 50%.
Embodiment 3
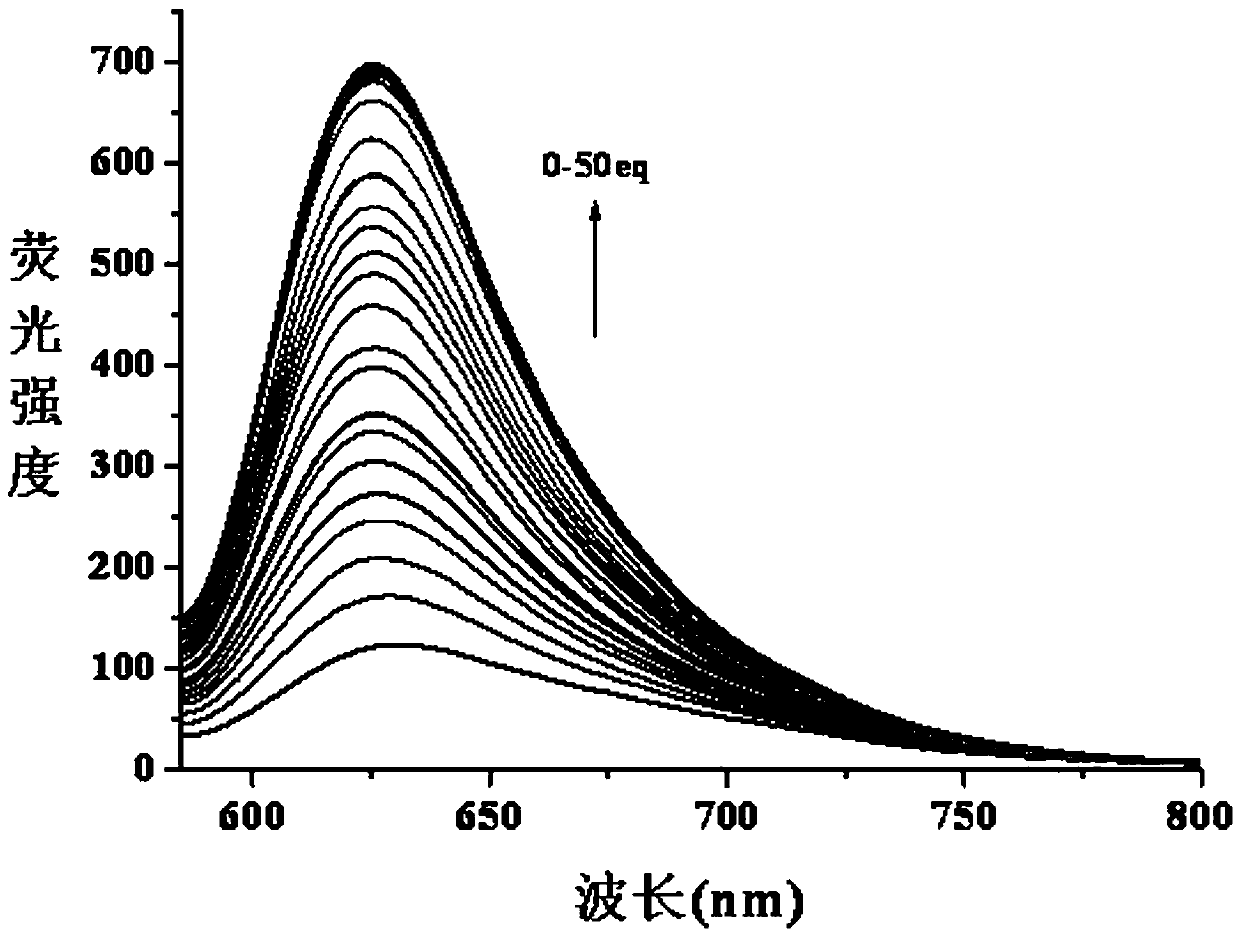
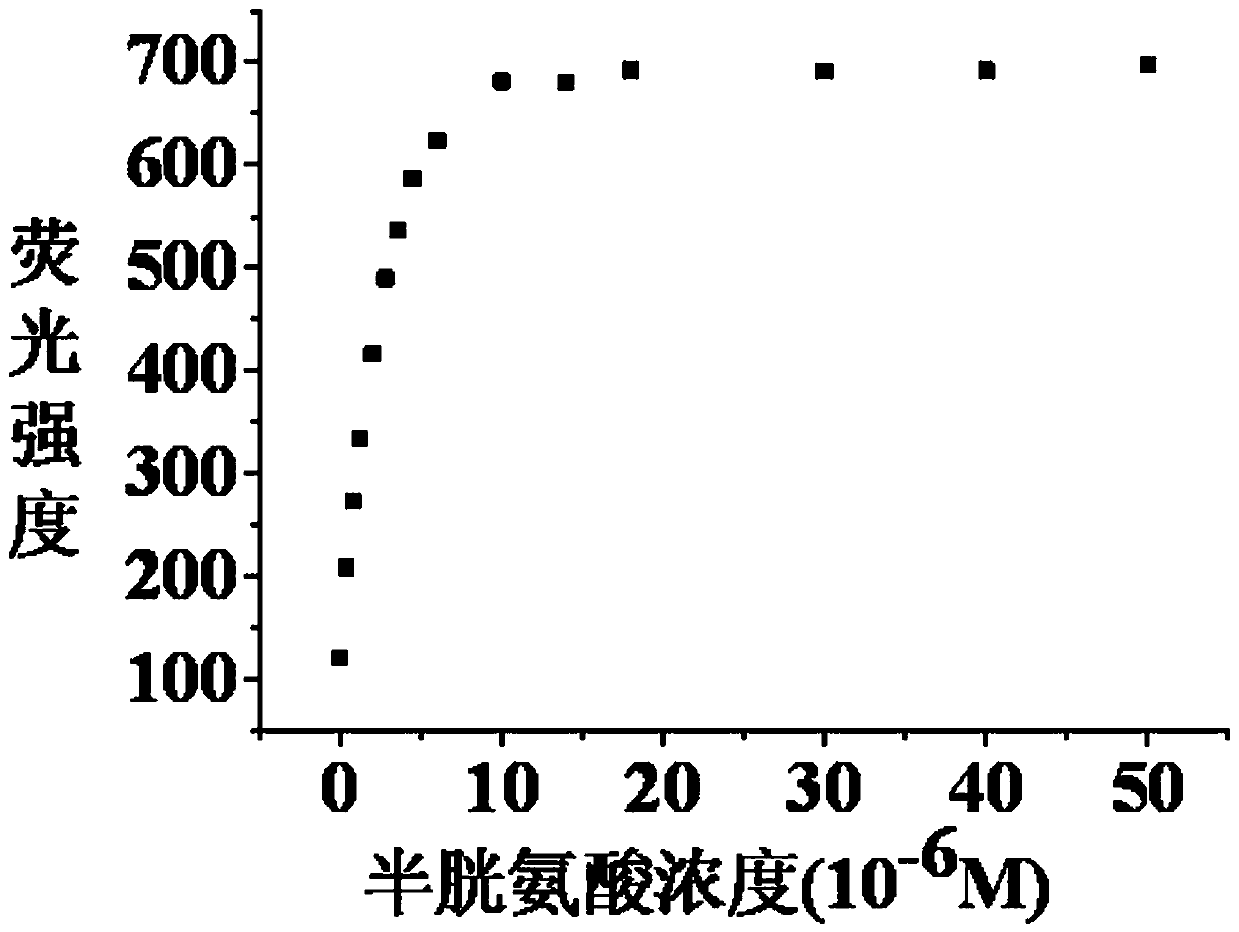
[0035] Example 3: The titration experiment of pH=7.4 fluorescent probe and cysteine: In the PBS buffer solution with pH=7.4, add the fluorescent probe with an initial concentration of 2 mM, so that the concentration of the fluorescent probe in the solution is 10 μM. Then, different amounts of biothiols with an initial concentration of 20 mM were added sequentially, so that the concentrations of biothiols in the solution were 2 μM, 4 μM, 6 μM, 8 μM, 10 μM, 12 μM, 14 μM, 16 μM, 18 μM, 20 μM, 24 μM, 28 μM, 32 μM, 36 μM, 40 μM, 45 μM, 50 μM, 60 μM, 80 μM, 100 μM, 120 μM, 140 μM, 160 μM, 180 μM, 200 μM, 250 μM, 300 μM, 500 μM, without adding cysteine as a control, let stand for 0.5 hours to fully react cysteine and fluorescent probe. The absorption and fluorescence spectra of cysteine under different concentrations were measured by absorption spectrometer and fluorescence spectrometer respectively. The excitation wavelength of the fluorescence spectrum was 570 nm, the emission...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com