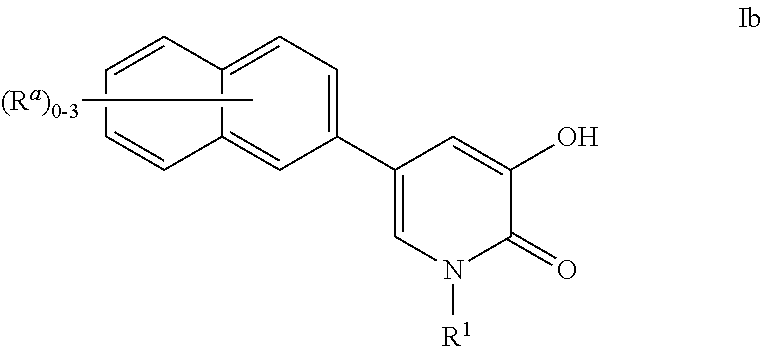
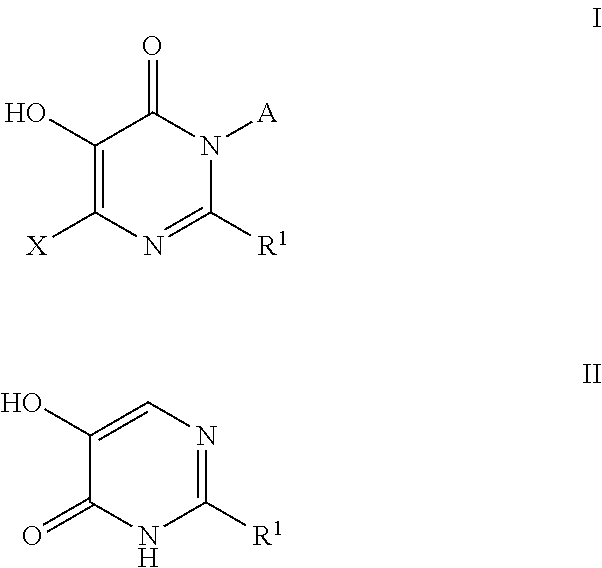
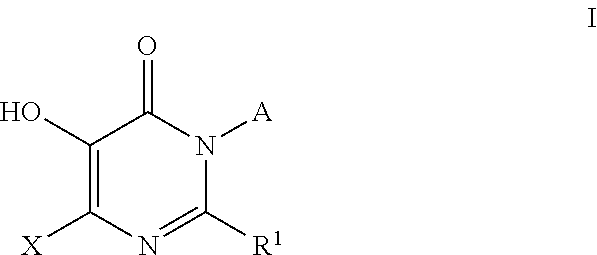
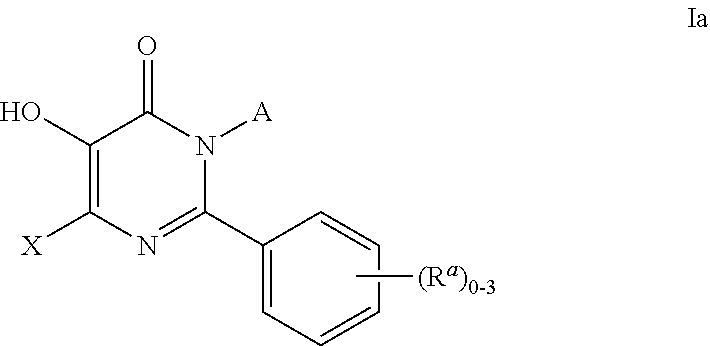
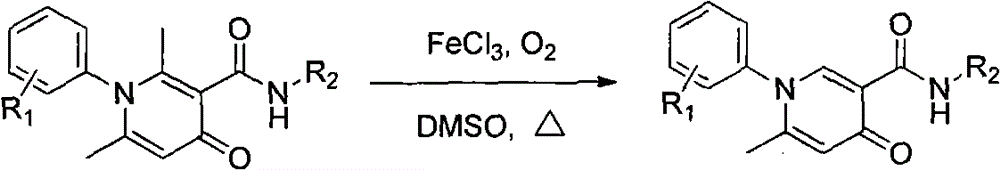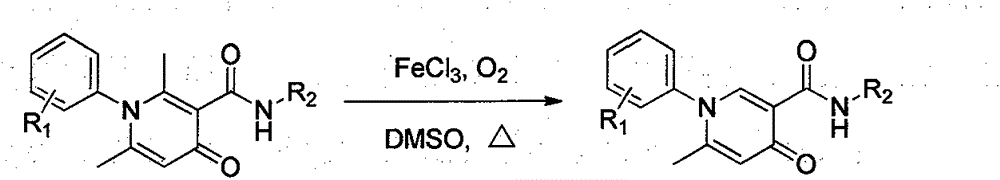Patents
Literature
41 results about "4-Pyridone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
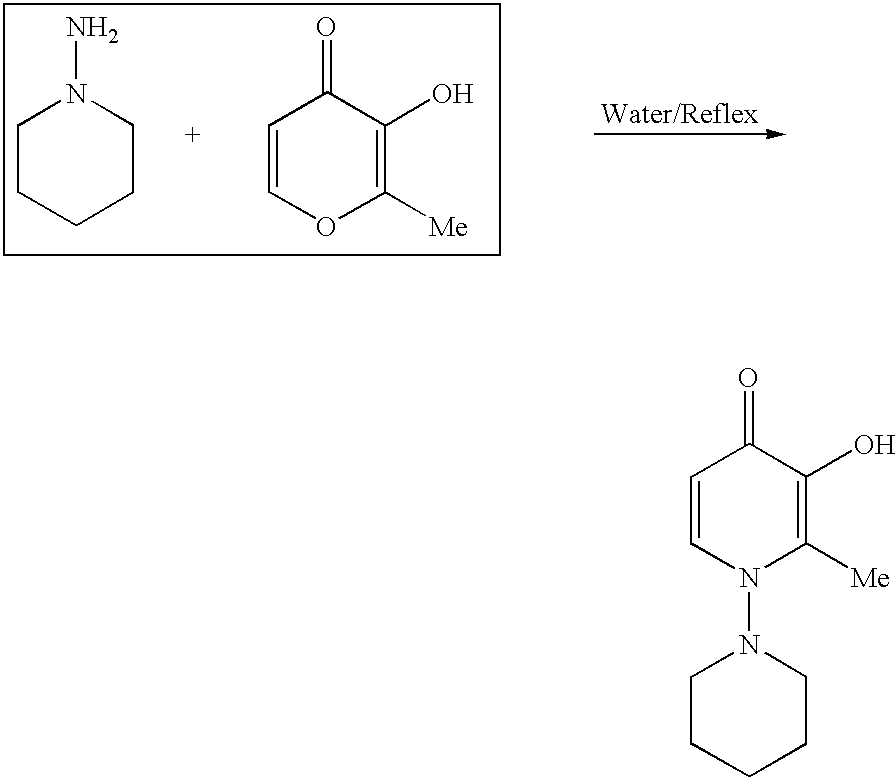
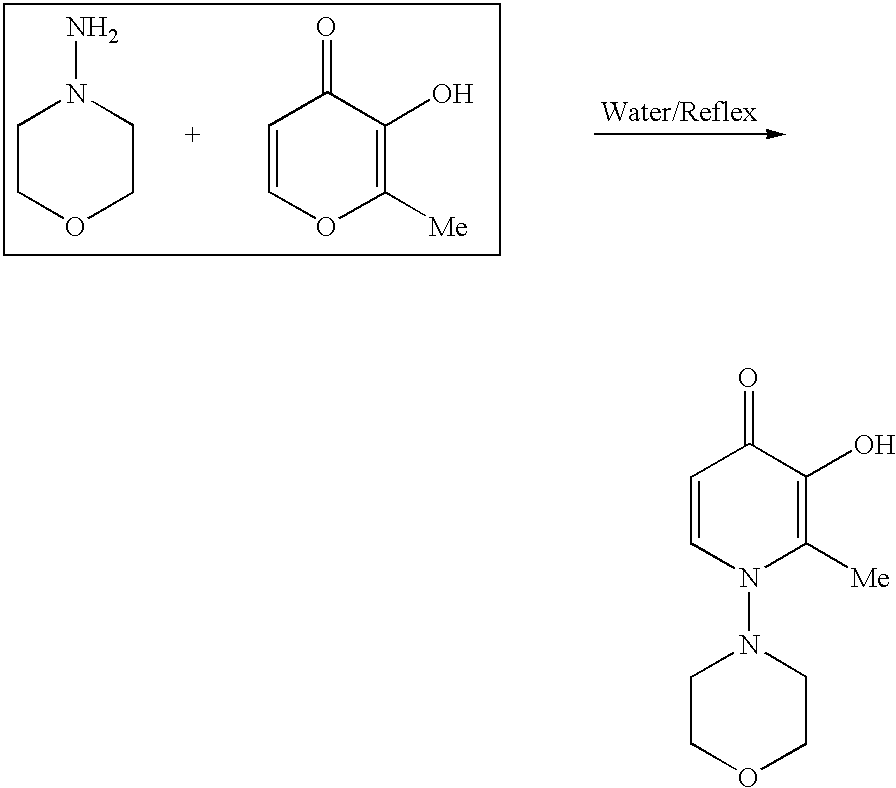
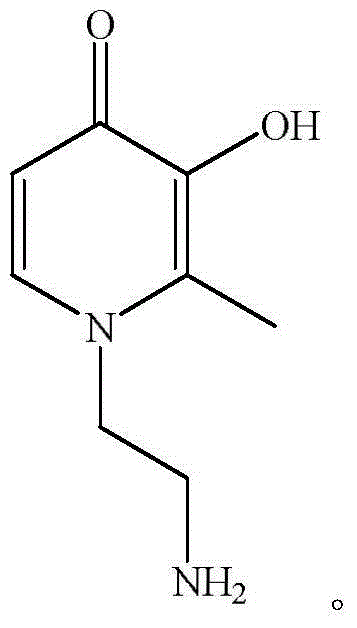
4-Pyridone is an organic compound with the formula C₅H₄NH(O). It is a colorless solid. The compound exists in equilibrium with a minor tautomer, pyridin-4-ol.
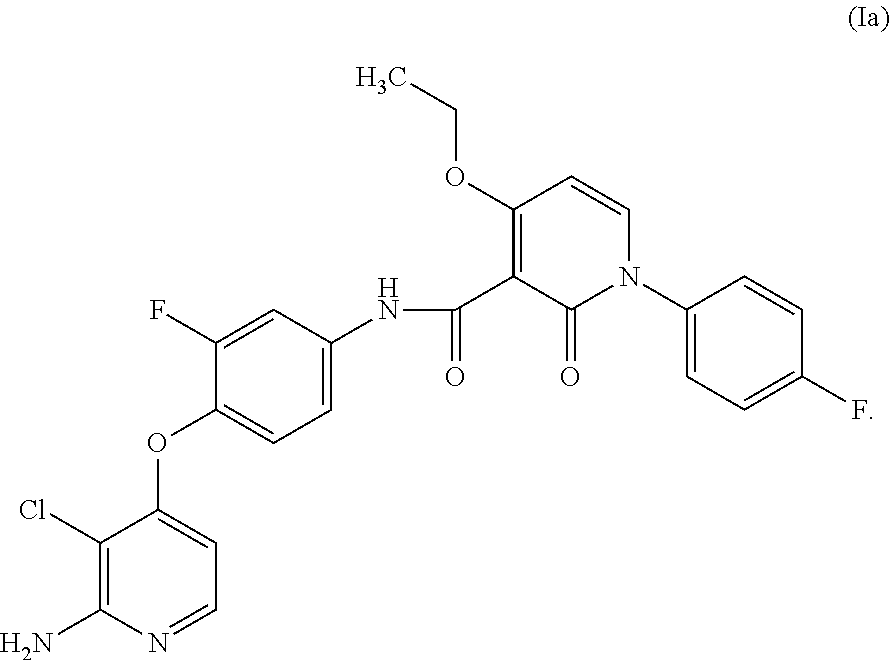
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
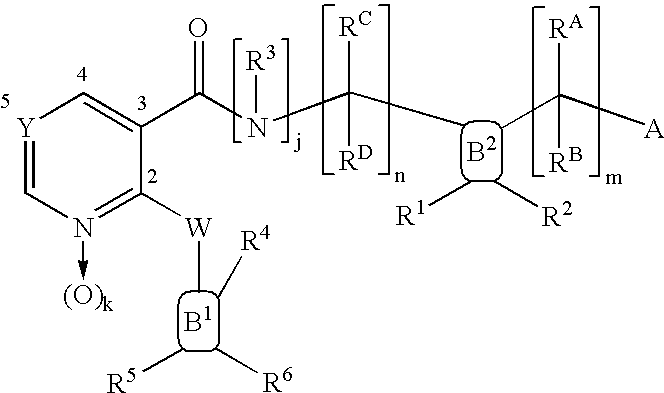
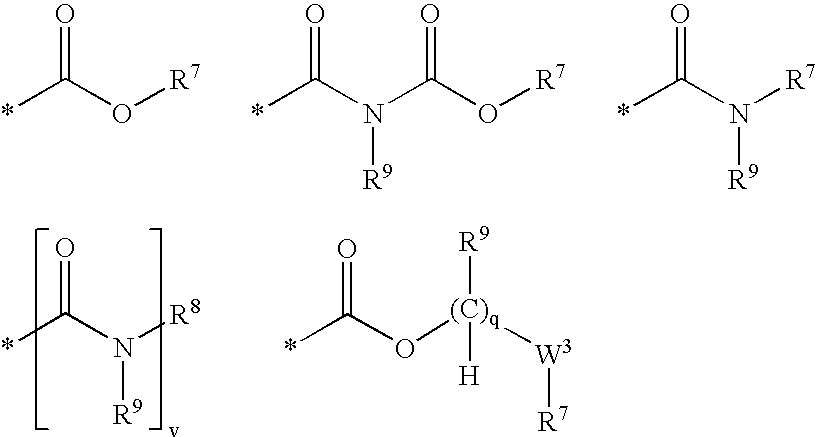
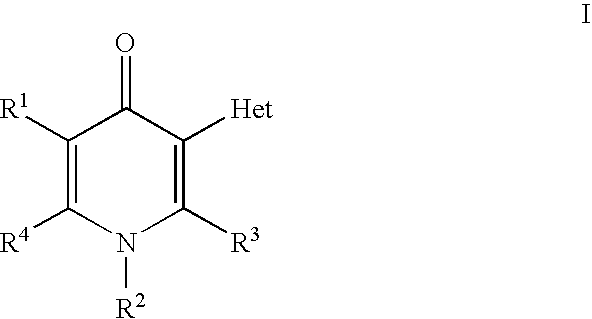
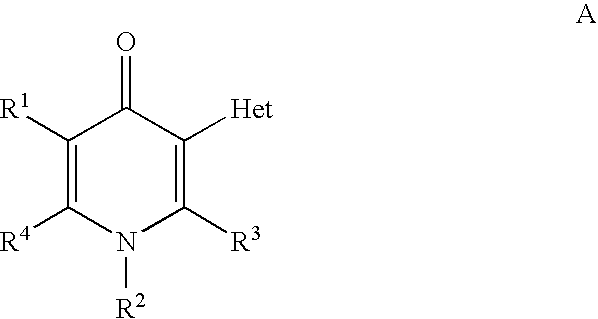
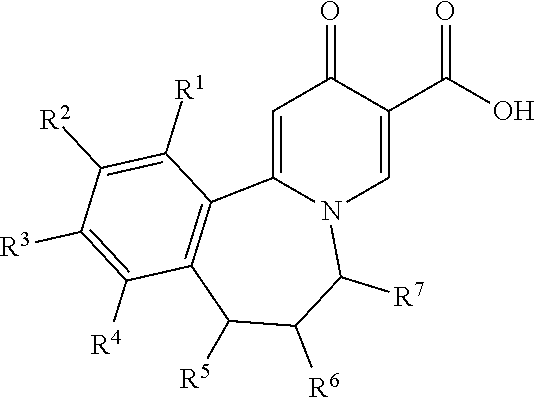
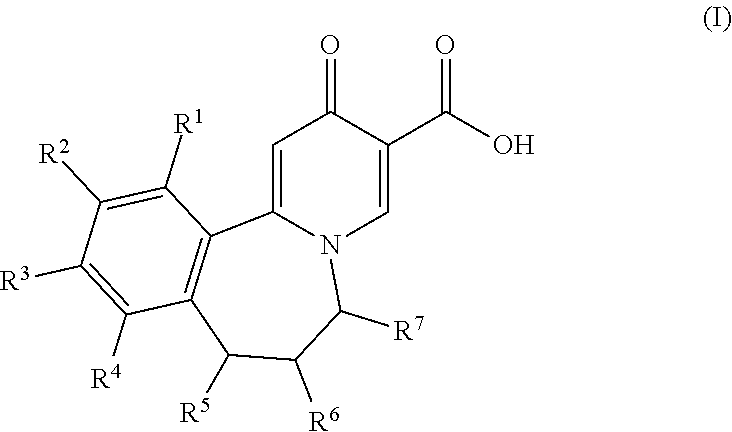
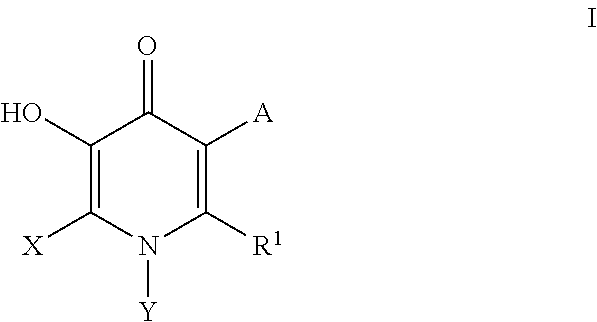
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
N-substituted 3-hydroxy-4-pyridinones and pharmaceuticals containing thereof
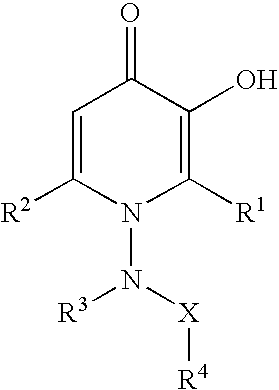
N-substituted 3-hydroxy-4-pyridinones and metal chelates, methods of preparing N-substituted 3-hydroxy-4-pyridinones and metal chelates, and pharmaceutical compositions containing new N-substituted 3-hydroxy-4-pyridinones and / or their metal chelates. Use of N-substituted 3-hydroxy-4-pyridinones and their metal chelates as pharmaceutical agents for the treatment of diseases, such as parasitic and viral infections, conditions associated with inflammation and infection, and conditions mediated by cell-proliferation or collagen formation.
Owner:LANTHEUS MEDICAL IMAGING INC
Compositions And Methods Related To Mitigating Aluminum And Ferric Precipitates In Subterranean Formations After Acidizing Operations
Trivalent-ion chelating agents may be useful in mitigating the formation aluminum and ferric precipitates in subterranean formations during acidizing operations. An acidizing operation may include introducing an acidizing fluid into a wellbore penetrating a subterranean formation, the acidizing fluid comprising an aqueous fluid, an acid source, and a trivalent-ion chelating agent, e.g., hydroxamates, 6,7-dihydroxyquinoline, 3-hydroxy-4-pyridinones, and any combination thereof. In some instances, trivalent-ion chelating agents with a pKa of less than about 7 may be particularly useful in acidizing operations.
Owner:HALLIBURTON ENERGY SERVICES INC
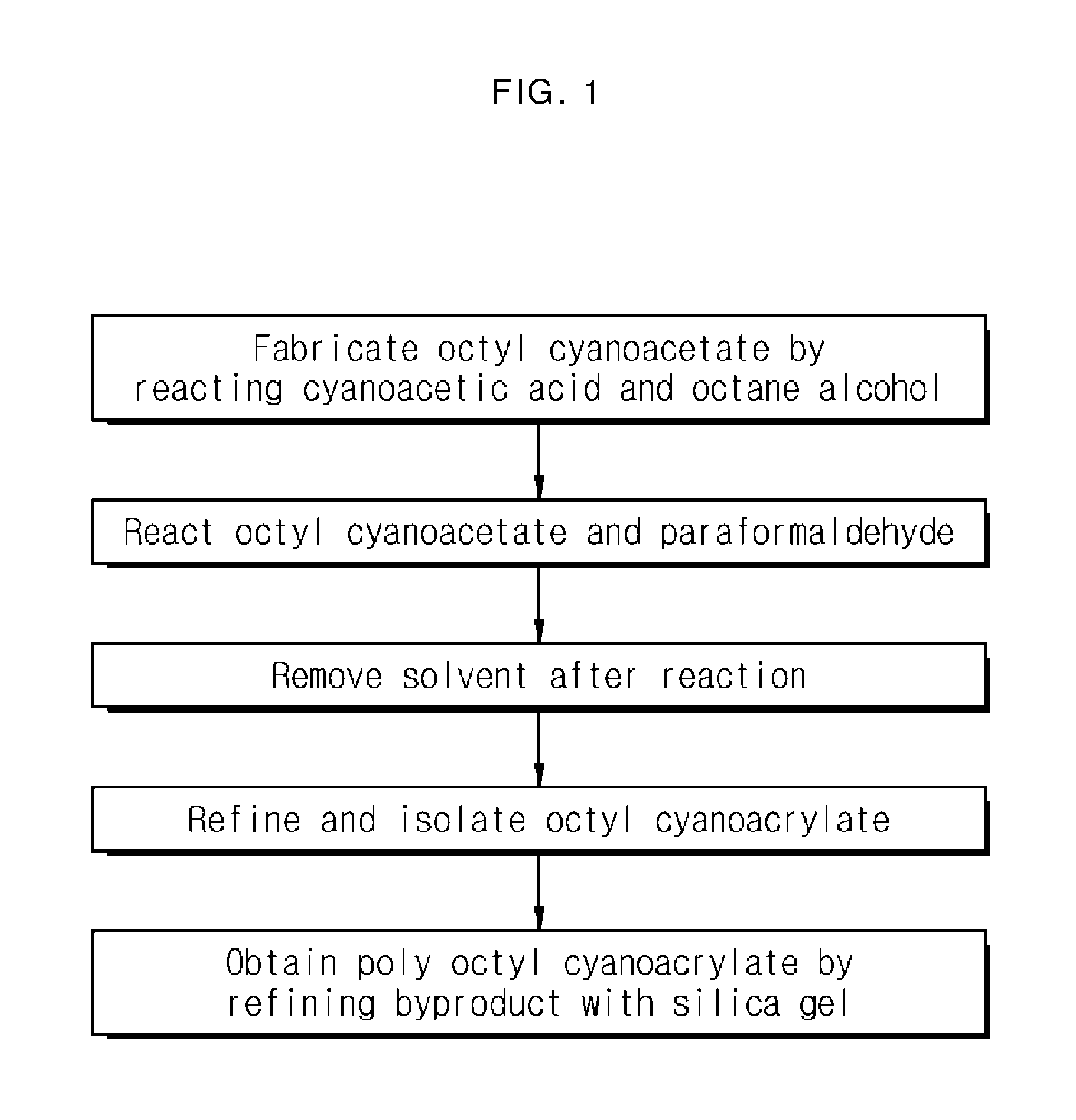
Cyanoacrylate-based bio-adhesive composition
InactiveUS20130225640A1Promote degradationGood antibacterial effectBiocideSurgical adhesivesAcetic acidBioadhesive
The present invention relates to a cyanoacrylate-based bio-adhesive composition, and aims to provide a cyanoacrylate-based bio-adhesive composition having an excellent biodegradability and anti-bacterial effect. The present invention provides a cyanoacrylate-based bio-adhesive composition having an enhanced biodegradability by using, as a thickening agent, poly octyl cyanoacrylate generated as a byproduct when preparing octyl cyanoacrylate, and having an excellent anti-bacterial effect by using 3,5-diiodo-4-pyridone-1-acetic acid as an anion stabilizer.
Owner:INTUITIVE MEDI CORP
Pyridinone derivatives against malaria
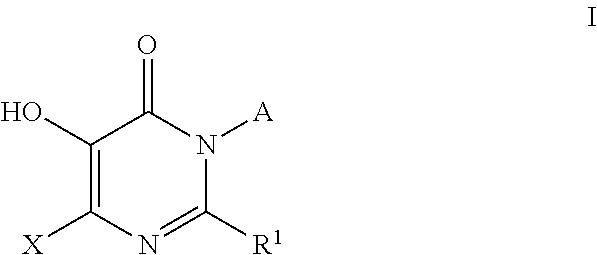
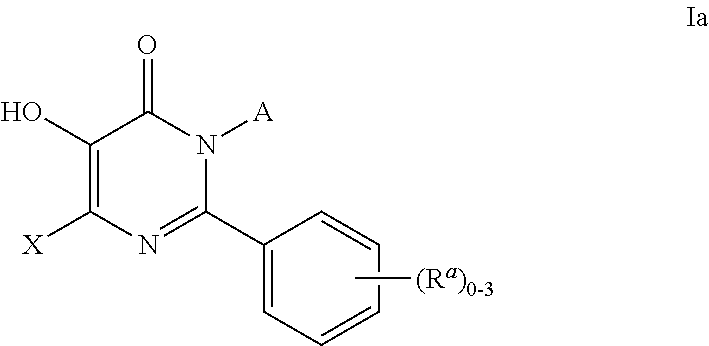
4-pyridone derivatives of Formula (I) and pharmaceutically acceptable derivatives thereof, processes for their preparation, pharmaceutical formulations thereof and their use in chemotherapy of certain parasitic infections such as malaria, are provided.
Owner:GLAXO GRP LTD
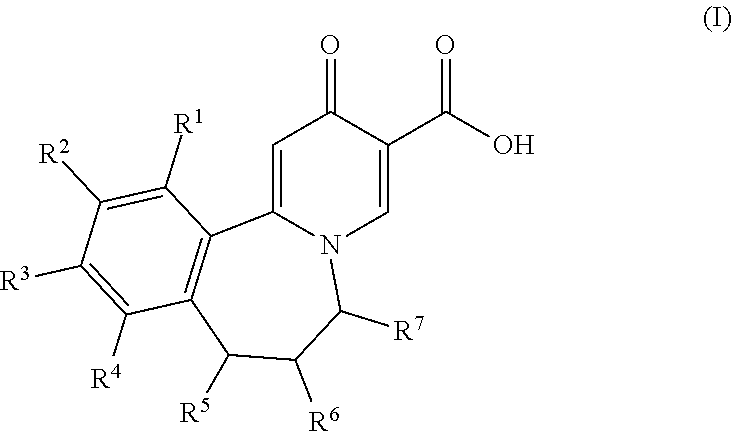
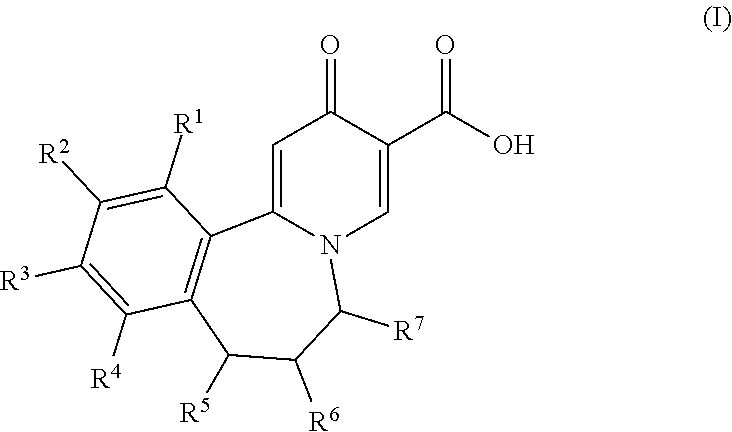
Novel tricyclic 4-pyridone-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis b virus infection
ActiveUS20180134705A1Treatment or prophylaxis of HBV infectionHigh activityOrganic chemistryAntiviralsCarboxylic acidHepatitis B virus
Owner:F HOFFMANN LA ROCHE & CO AG
Inhibitors of catechol o-methyl transferase and their use in the treatment of psychotic disorders
InactiveUS20130005744A1Improve negative symptomsGood effectBiocideSenses disorderDiseaseCatechol-O-methyl transferase
The present invention relates to 4-pyridinone compounds which are inhibitors of catechol O-methyltransferase (COMT), and are useful in the treatment and prevention of neurological and psychiatric disorders and diseases in which COMT enzyme is involved. The present invention also relates to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which COMT is involved.
Owner:MERCK SHARP & DOHME LLC
Inhibitors of catechol o-methyl transferase and their use in the treatment of psychotic disorders
The present invention relates to 4-pyridinone compounds which are inhibitors of catechol O-methyltransferase (COMT), and are useful in the treatment and prevention of neurological and psychiatric disorders and diseases in which COMT enzyme is involved. The present invention also relates to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which COMT is involved.
Owner:MERCK SHARP & DOHME CORP
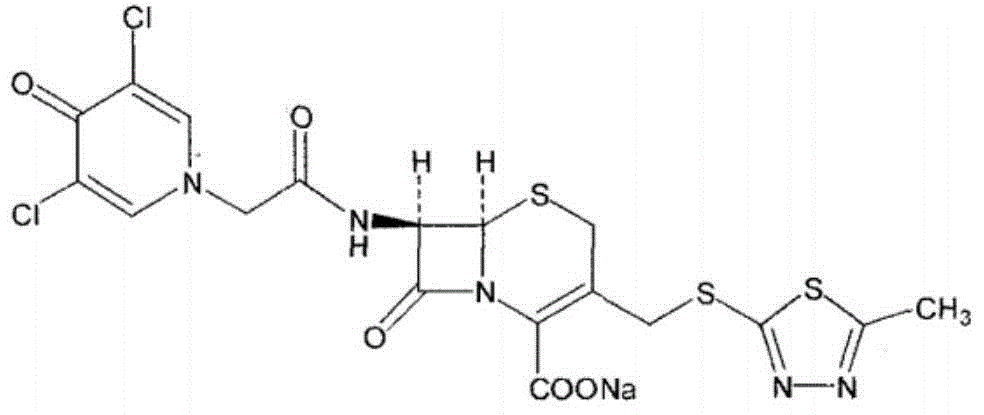
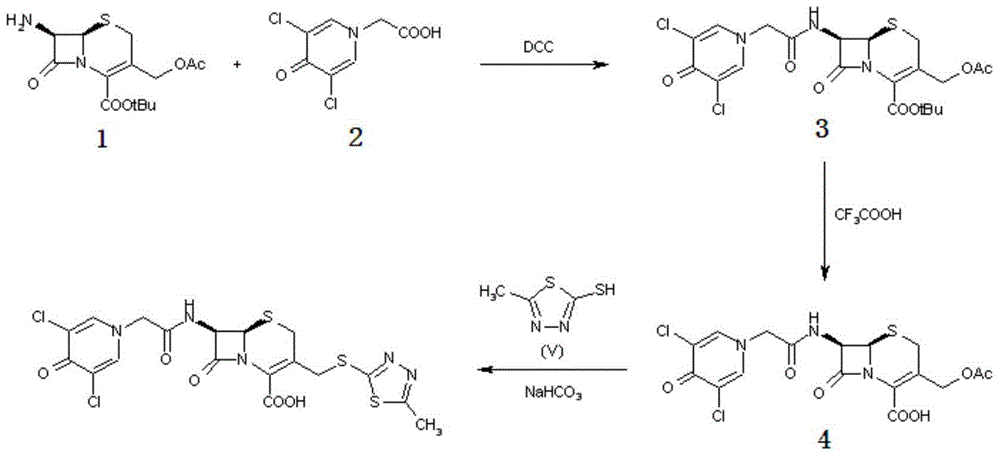
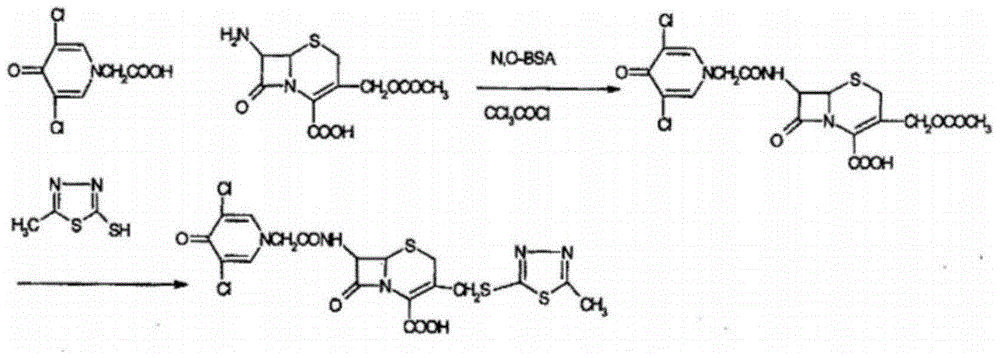
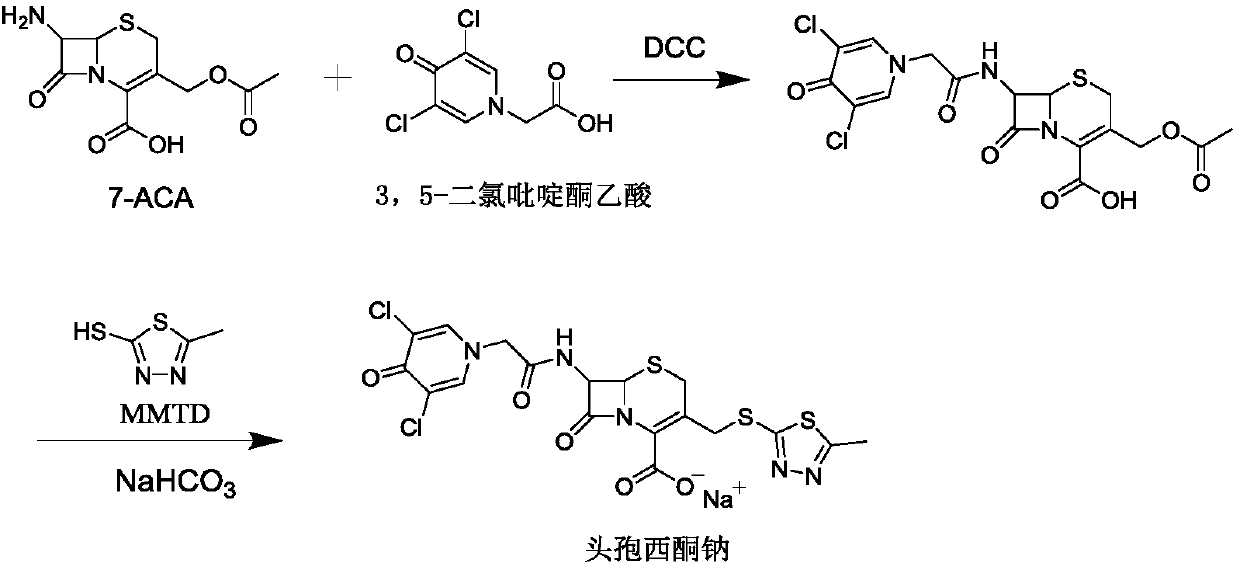
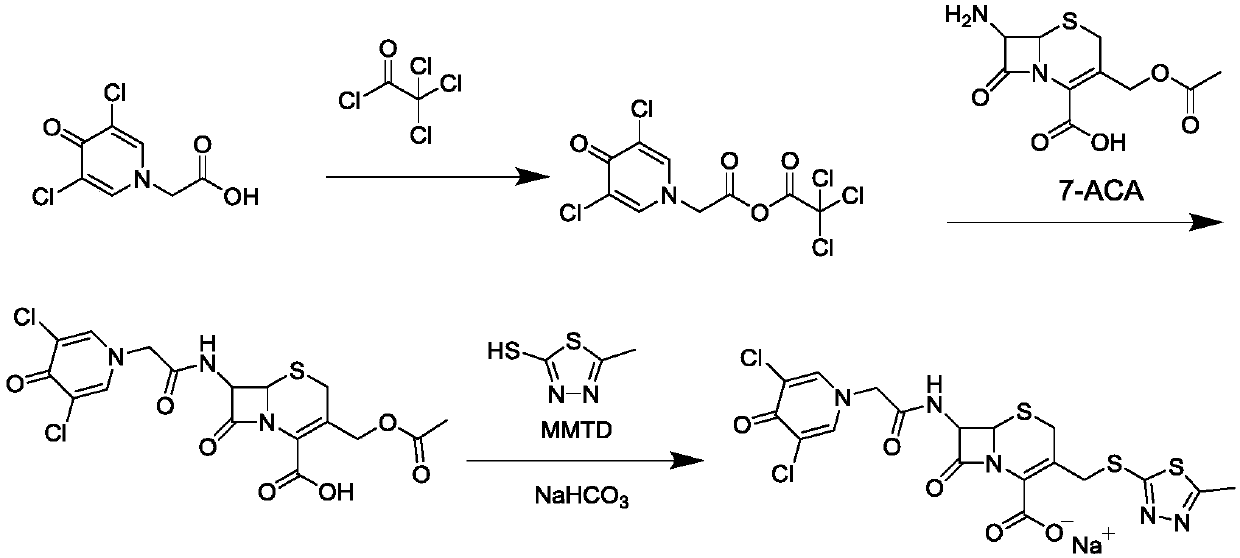
A kind of synthesis technique of cephalosporin anti-infective drug
ActiveCN105017285BNo pollution in the processThe synthesis process is simpleOrganic chemistryAcetic acidThiol
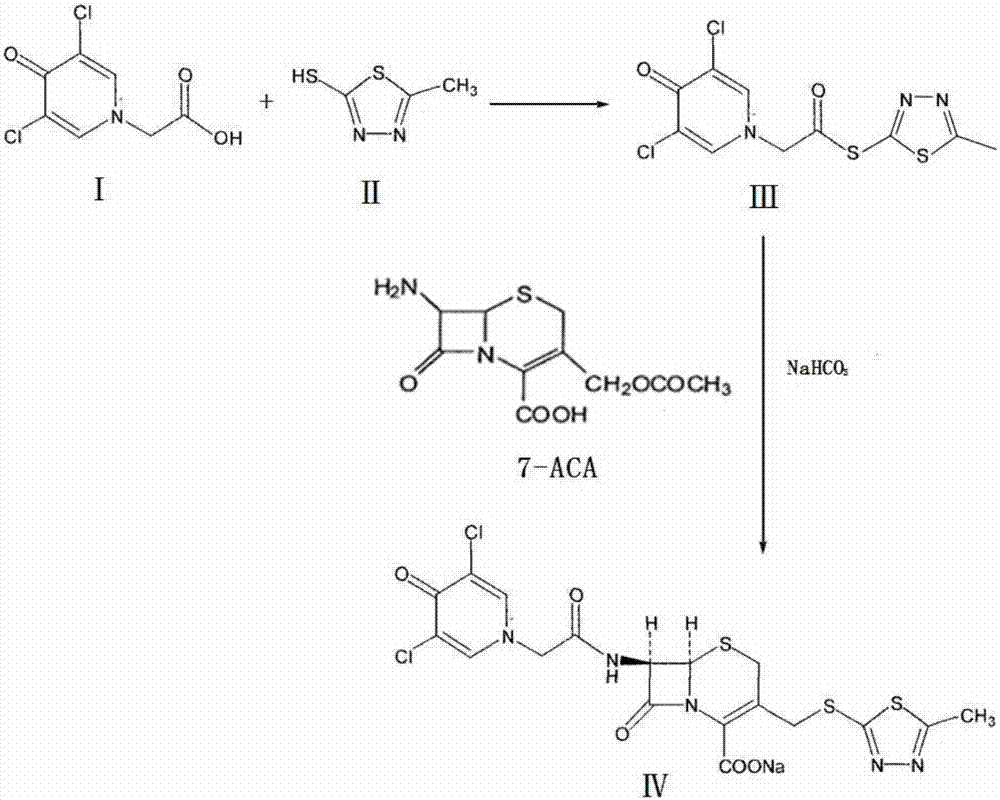
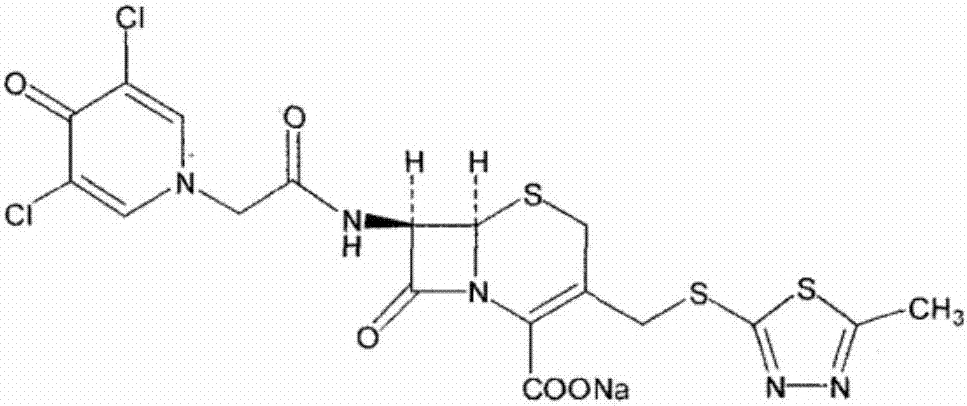
The invention relates to a synthetic process of a novel cephalosporin anti-infective drug. A thioester compound (formula III) synthesized by adopting 3,5-dichloro-4-pyridone-1-acetic acid and 2-thiol-5-methyl-1,3,4-thiadiazole as raw materials is directly reacted with 7-ACA in one step to successfully prepare cefazedone sodium. According to the synthetic process disclosed by the invention, the operating steps are simplified, so that the production conditions are milder, the generation of byproducts is reduced, and the production technology is simpler; and moreover, the product yield and purity are greatly improved, the cost is reduced, the environmental pollution is little, and the synthetic process is higher in environmental protection, so that the process accords with the requirement of industrialization.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
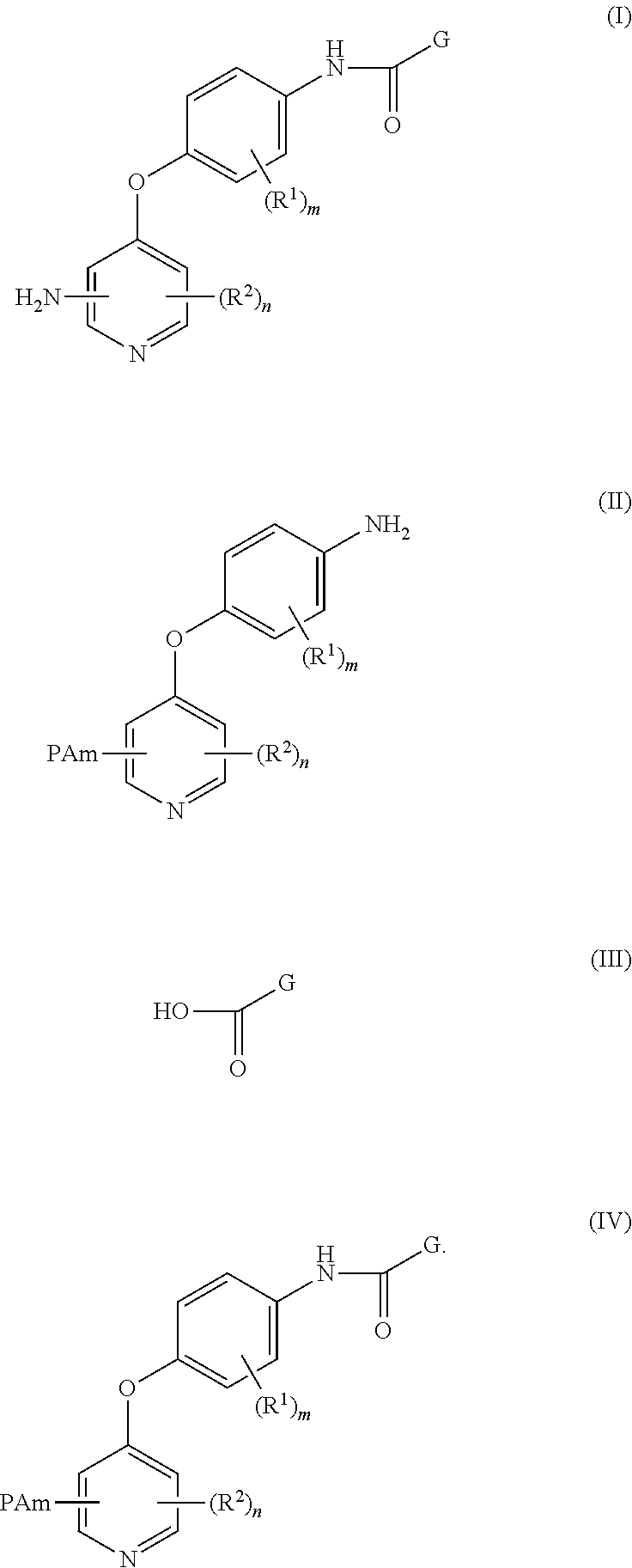
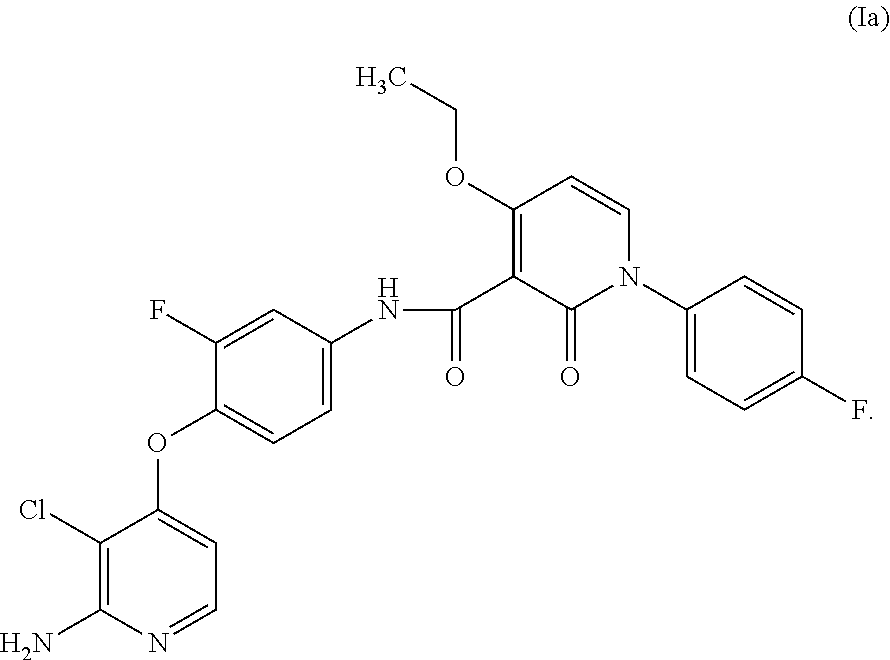
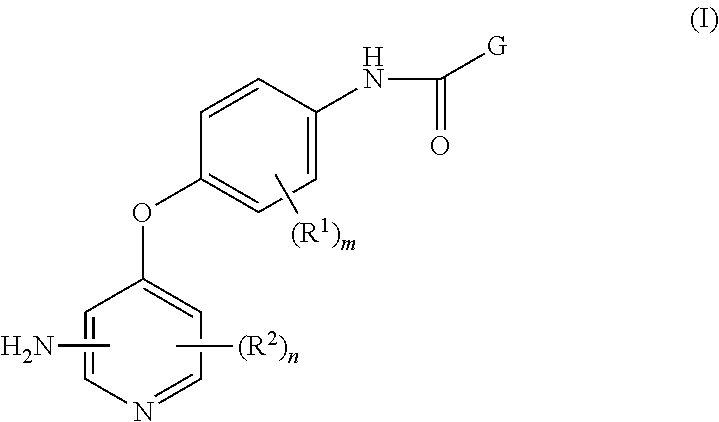
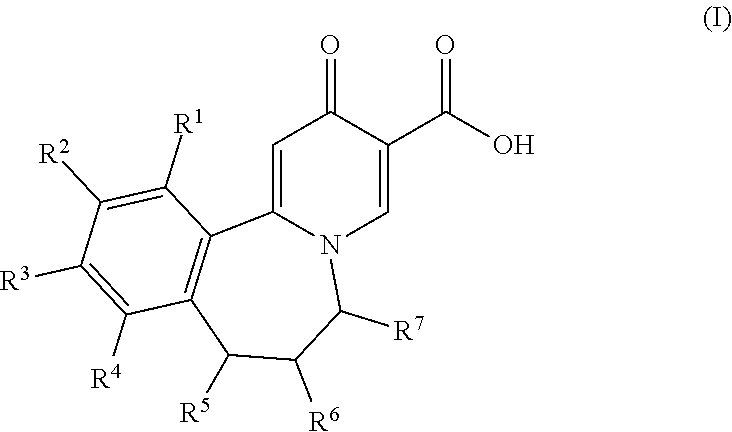
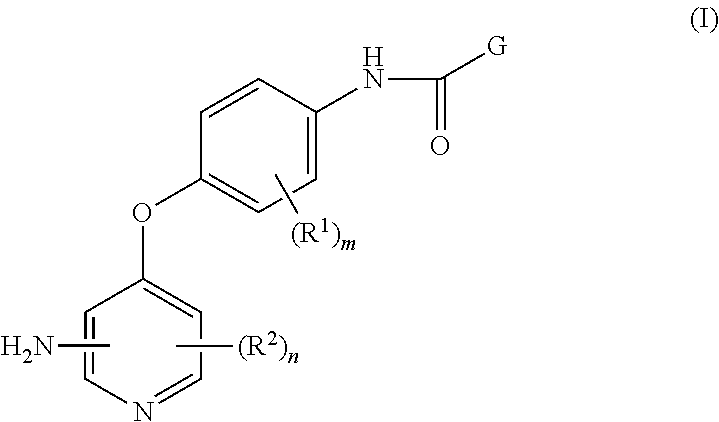
4-pyridinone compounds and their use for cancer
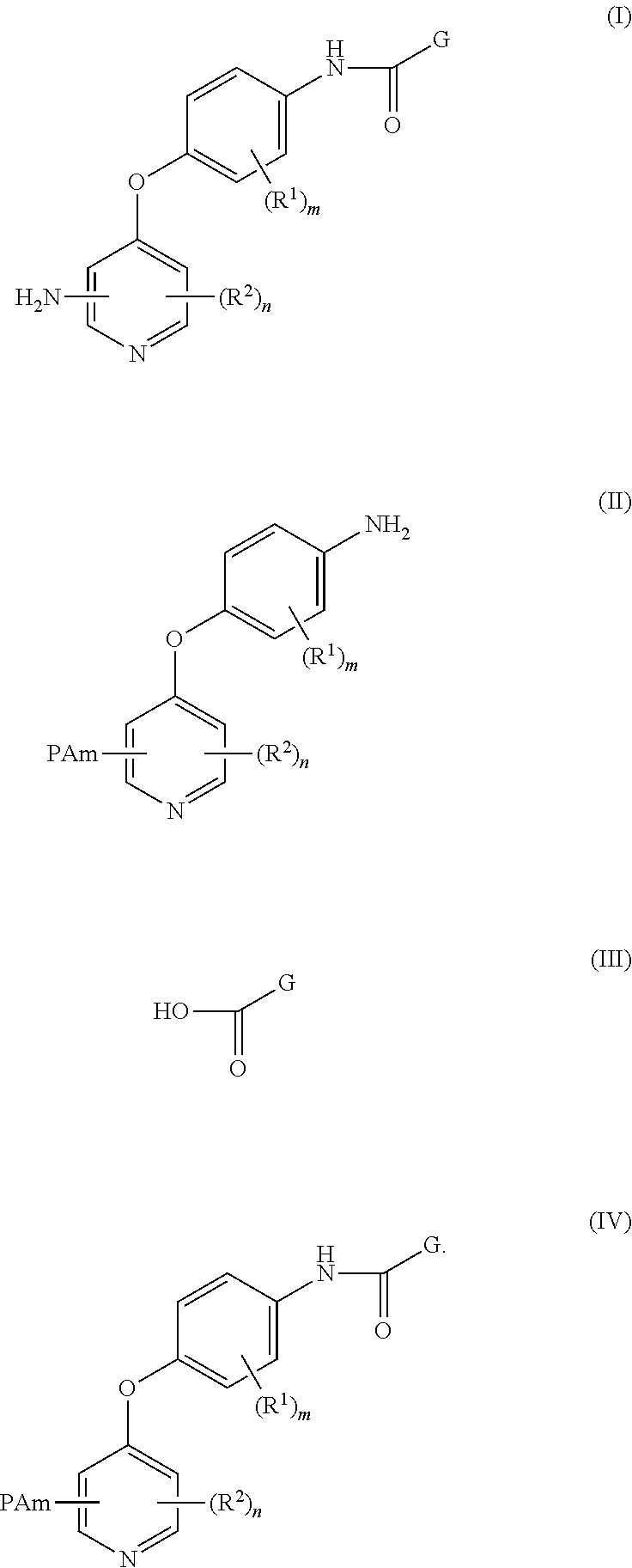
Disclosed herein is a process for preparing a compound of Formula (I), comprising the steps of: (a) reacting an aniline compound of Formula (II) and an carboxylic acid compound of Formula (III) or an activated carboxylic acid compound thereof, to provide a compound of Formula (IV); and (b) converting said protected amine group attached to said compound of Formula (IV) to an amine group to provide said compound of Formula (I); wherein PAm is a protected amine group. Processes to prepare the compounds of Formulae (II), (III), and (IV) are also disclosed.
Owner:BRISTOL MYERS SQUIBB CO
Synthetic process of novel cephalosporin anti-infective drug
ActiveCN105017285ANo pollution in the processThe synthesis process is simpleOrganic chemistryAcetic acidThiol
The invention relates to a synthetic process of a novel cephalosporin anti-infective drug. A thioester compound (formula III) synthesized by adopting 3,5-dichloro-4-pyridone-1-acetic acid and 2-thiol-5-methyl-1,3,4-thiadiazole as raw materials is directly reacted with 7-ACA in one step to successfully prepare cefazedone sodium. According to the synthetic process disclosed by the invention, the operating steps are simplified, so that the production conditions are milder, the generation of byproducts is reduced, and the production technology is simpler; and moreover, the product yield and purity are greatly improved, the cost is reduced, the environmental pollution is little, and the synthetic process is higher in environmental protection, so that the process accords with the requirement of industrialization.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Substituted 3-hydroxy-4-pyridone derivative
This invention provides compounds having antiviral activities especially inhibiting activity for influenza virus, more preferably provides substituted 3-hydroxy-4-pyridone derivatives having cap-dependent endonuclease inhibitory activity.
Owner:SHIONOGI & CO LTD
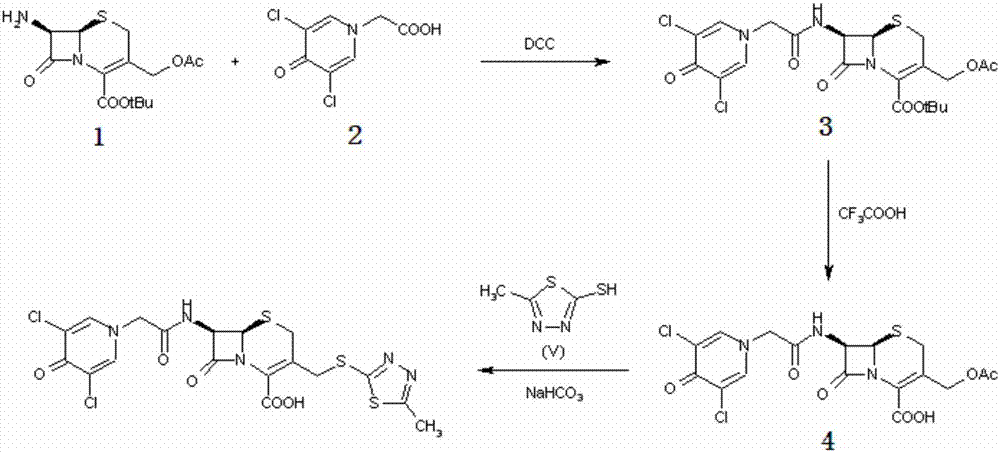
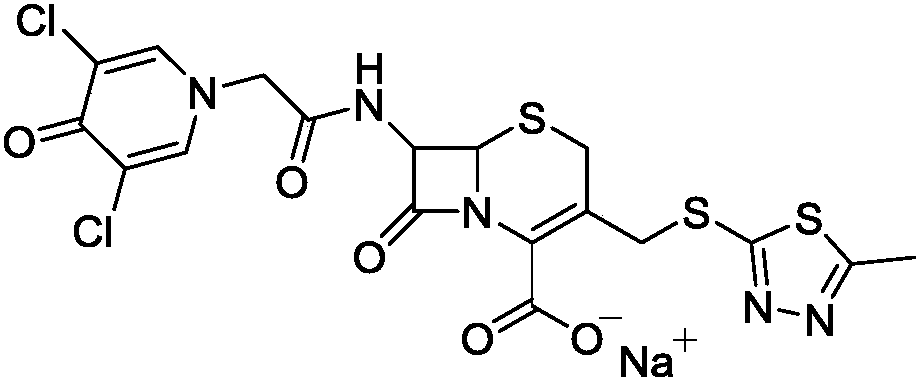
Synthetic method of cefazedone sodium
ActiveCN109553630AImprove acylation activityGuaranteed stabilityOrganic chemistryAcetic acidCefazedone sodium
The invention belongs to the technical field of medicines, and discloses a synthetic method of cefazedone sodium. Active ester is synthesized by taking 3,5-dichloro-4-pyridone-1-acetic acid and 2,2'-dithiobis(benzothiazole) as raw materials, then the active ester reacts with an intermediate generated from 7-aminocephalosporanic acid and mercaptoterazole, and the cefazedone sodium is obtained through salifying. According to the invention, a mixed solvent is used in 3-position substitution, so that the reaction is more stable and soft, and generation of by-products is reduced; the active ester is used in an acylation reaction, so that activity is high, and a 7-position acylation reaction is facilitated; and the yield and purity of the cefazedone sodium are relatively high.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
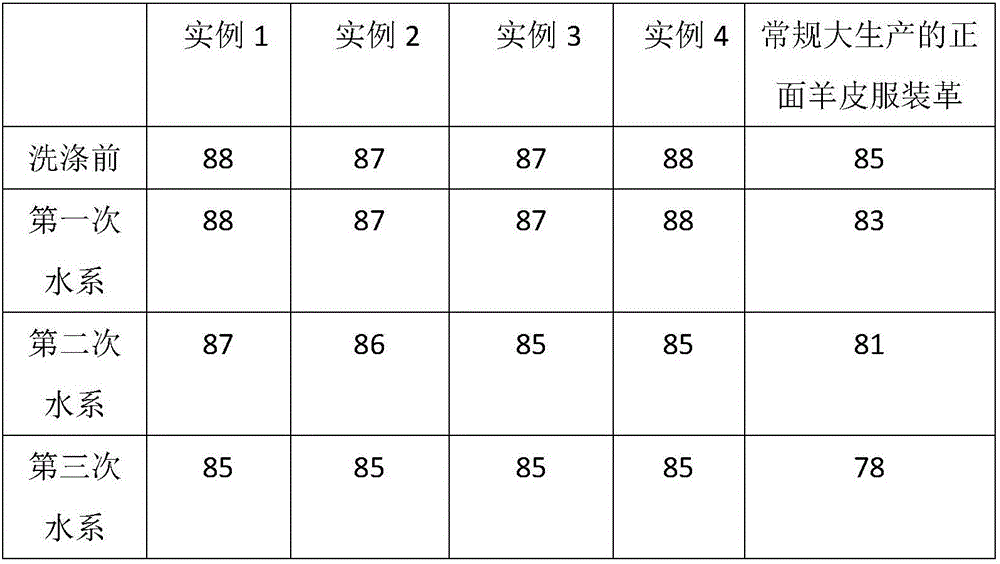
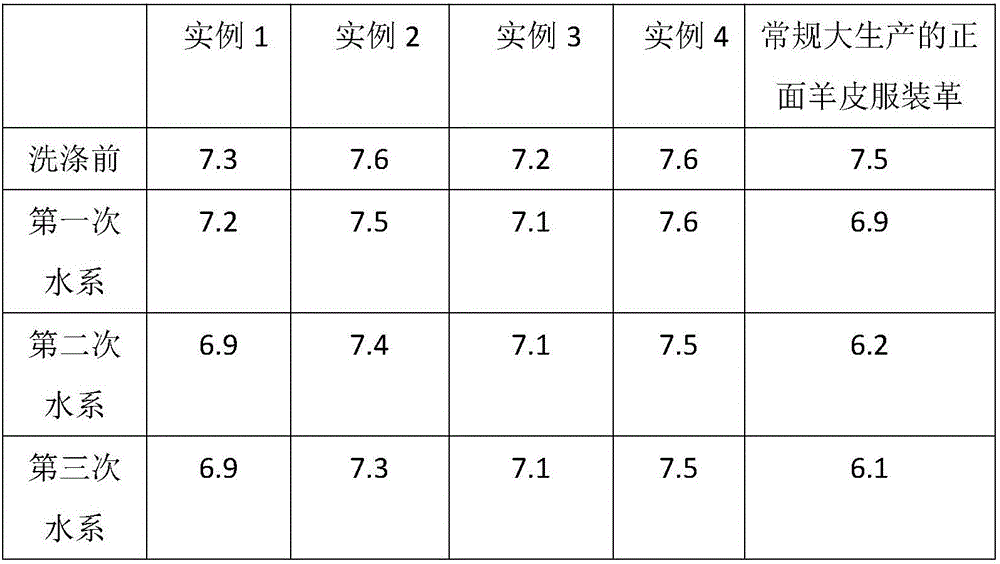
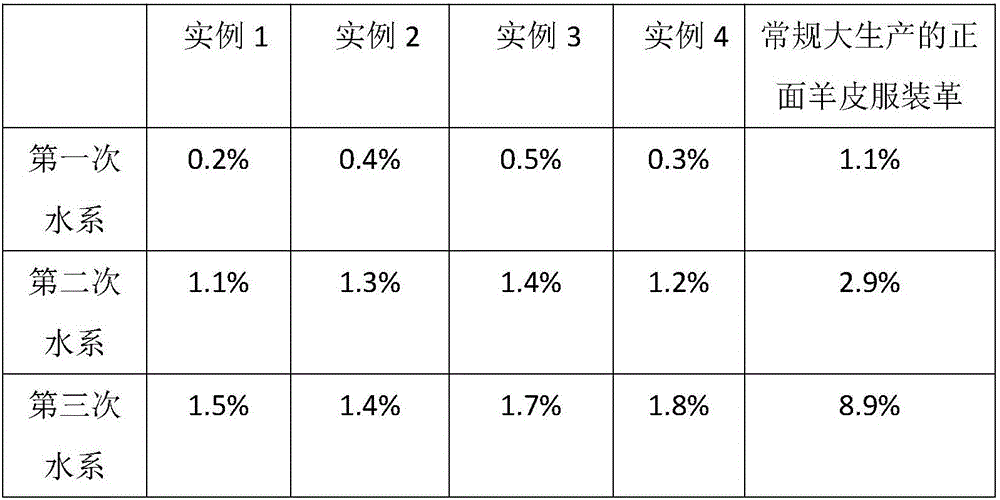
Processing technology for washable and environment-friendly clothing leather
ActiveCN106282439AImprove mechanical propertiesPromote absorptionTanning treatmentLeather/skins/hides/pelt chemical treatment apparatusEthylenediamineNaringin
The invention discloses a processing technology for washable and environment-friendly clothing leather. The processing technology is characterized in that chrome-free tan, sodium ethylene diamine tetracetate, 1,2-dimethyl-3-hydroxy-4-pyridone, 2-hydroxy propane-1,2,3-tricarboxylic acid sodium, 2-phosphonic acid butane-1,2,4-tricarboxylic acid sodium salt and amino trimethylene phosphonic acid are used for fixing an aluminum tanning agent, and the washing resistance of the clothing leather is improved; and meanwhile, the mechanical performance of the clothing leather is improved by adopting naringin, N-naphthalene-1-radical-3-hydroxyl naphthalene-2-formamide and glycine, so that the washable and environment-friendly clothing leather is prepared.
Owner:湖南永霏特种防护用品有限公司
Tricyclic 4-pyridone-3-carboxylic acid derivatives for the treatment and prophylaxis of hepatitis B virus infection
ActiveUS10336751B2Treatment or prophylaxis of HBV infectionHigh activityOrganic chemistryAntiviralsCarboxylic acidHepatitis B virus
Owner:F HOFFMANN LA ROCHE & CO AG
Compositions and methods related to mitigating aluminum and ferric precipitates in subterranean formations after acidizing operations
Trivalent-ion chelating agents may be useful in mitigating the formation aluminum and ferric precipitates in subterranean formations during acidizing operations. An acidizing operation may include introducing an acidizing fluid into a wellbore penetrating a subterranean formation, the acidizing fluid comprising an aqueous fluid, an acid source, and a trivalent-ion chelating agent, e.g., hydroxamates, 6,7-dihydroxyquinoline, 3-hydroxy-4-pyridinones, and any combination thereof. In some instances, trivalent-ion chelating agents with a pKa of less than about 7 may be particularly useful in acidizing operations.
Owner:HALLIBURTON ENERGY SERVICES INC
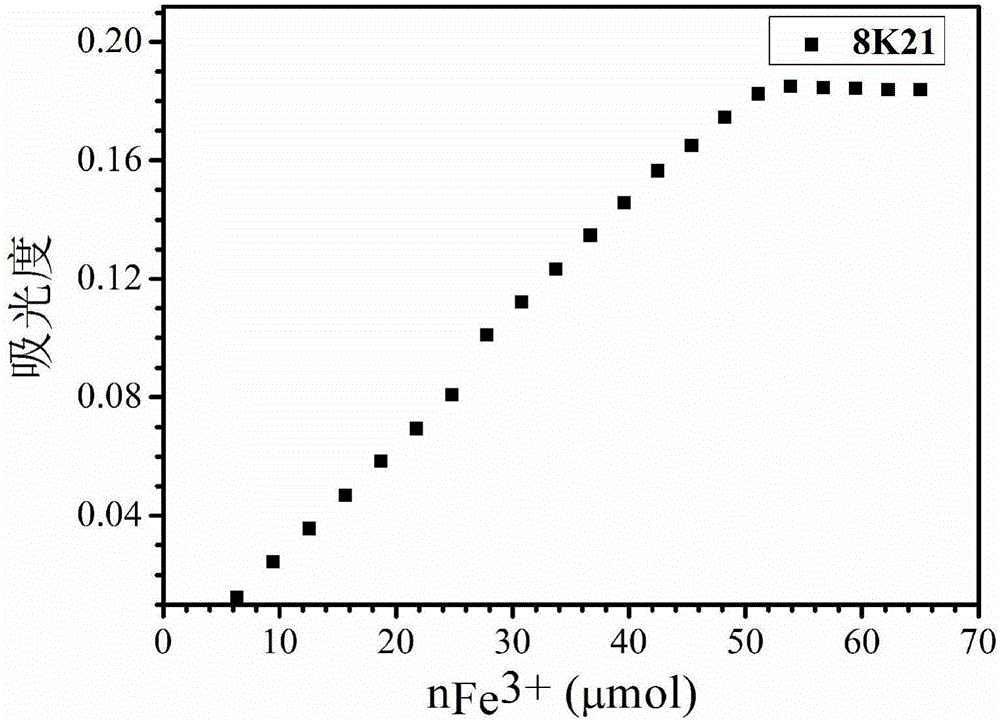
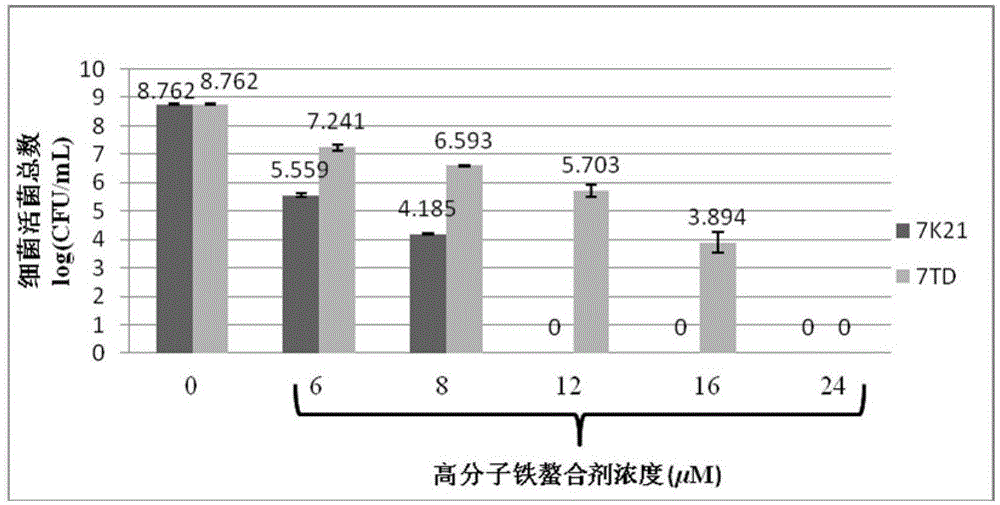
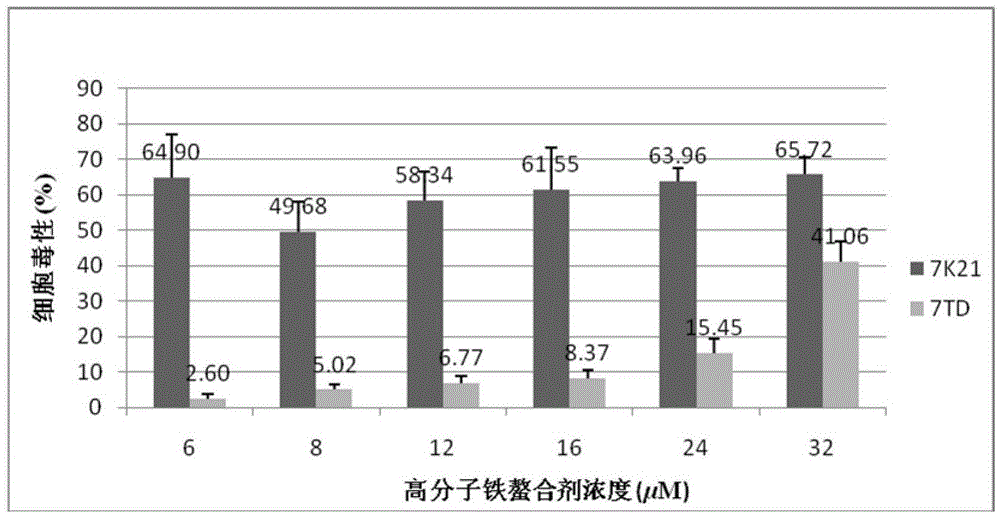
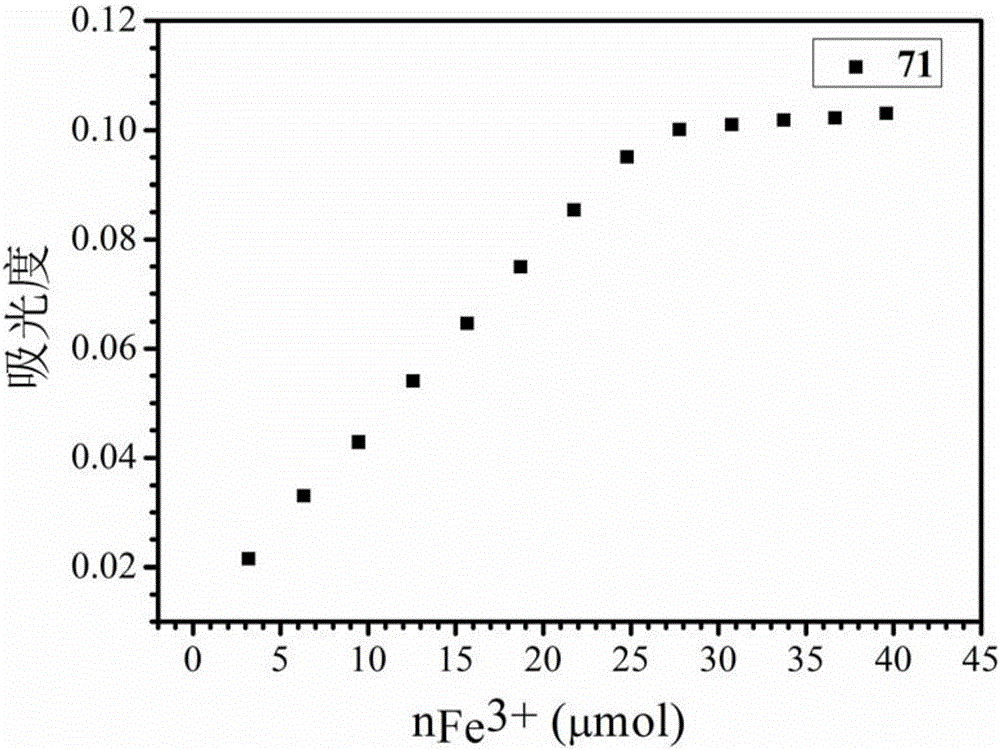
Use of 3-hydroxy-4-pyridone high-molecular iron chelating agent
ActiveCN105030819AHas antibacterial propertiesGood antibacterial effectAntibacterial agentsOrganic active ingredientsSide effectIron Chelating
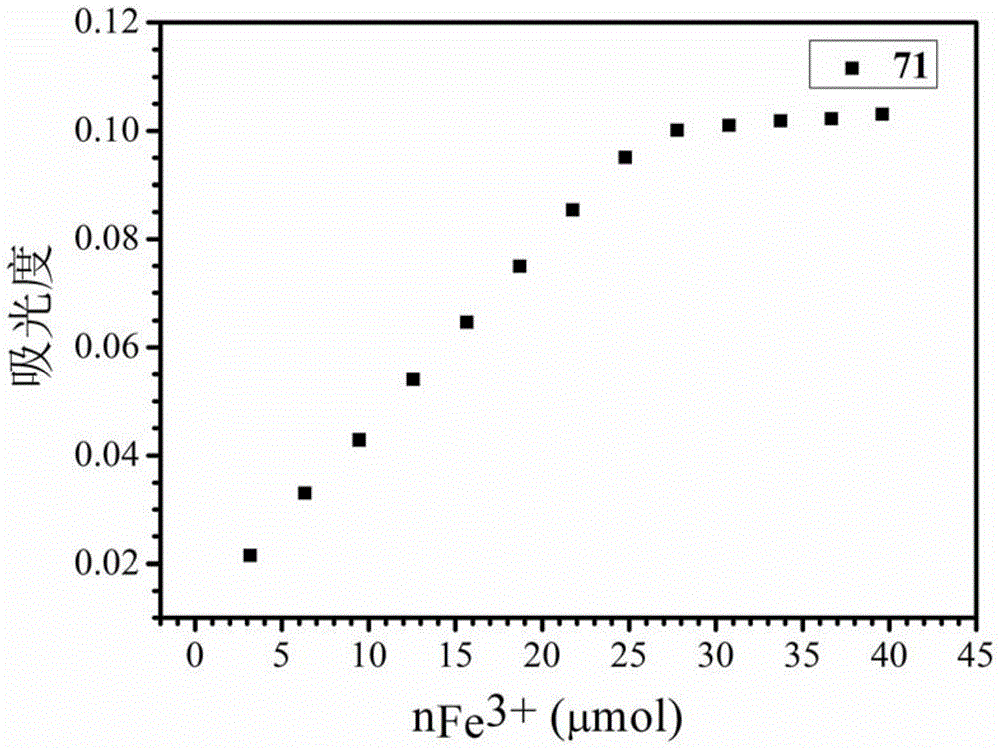
The invention discloses a use of a 3-hydroxy-4-pyridone high-molecular iron chelating agent. The 3-hydroxy-4-pyridone high-molecular iron chelating agent has antibacterial effects and can be used as an antibacterial material. The 3-hydroxy-4-pyridone high-molecular iron chelating agent has excellent effects of resisting methicillin-resistant staphylococcus aureus. According to a mechanism of cation simulative antibacterial peptide formation from tertiary amine or secondary amine positive ion structures in a high-molecular iron chelating agent, interaction between a positive charge area and a negative charge area on a cell membrane, and iron chelating effects of an iron chelating agent, the normal growth environment around bacteria is destroyed and bacterium growth is inhibited so that bacteria cannot normally grow. The 3-hydroxy-4-pyridone high-molecular iron chelating agent cannot be absorbed by skin because of macro-molecule characteristics, does not produce toxic or side effects and can be used as an antibacterial material with dual antibiosis effects.
Owner:UNIV OF SCI & TECH OF CHINA
Inhibitors of catechol O-methyl transferase and their use in the treatment of psychotic disorders
The present invention relates to 4-pyridinone compounds which are inhibitors of catechol O-methyltransferase (COMT), and are useful in the treatment and prevention of neurological and psychiatric disorders and diseases in which COMT enzyme is involved. The present invention also relates to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which COMT is involved.
Owner:MERCK SHARP & DOHME CORP
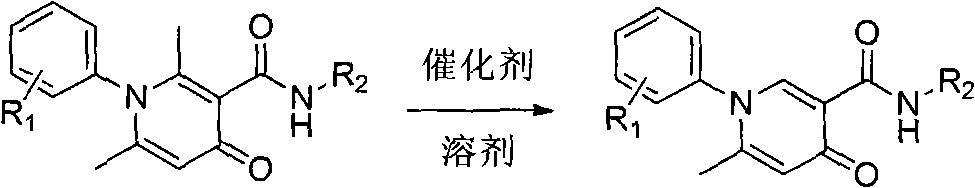
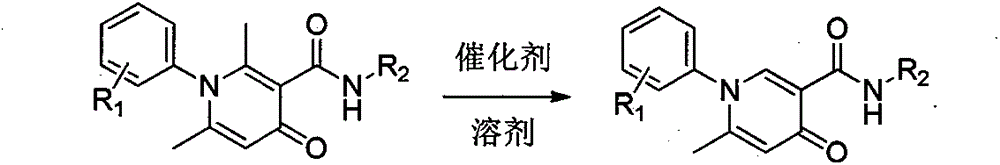
Simple and effective method for high-selectivity removal of alpha-monomethyl of 2,6-dimethyl-4-pyridone derivative
The invention discloses a simple and effective method for high-selectivity removal of an alpha-monomethyl of a 2,6-dimethyl-4-pyridone derivative. The simple and effective method comprises that an alpha-monomethyl of a 2,6-dimethyl-4-pyridone derivative is removed by oxygen as an oxidizing agent in the presence of anhydrous ferric chloride as a catalyst with heating; and after the reaction, cooling, extraction and separation processes are carried out so that a 6-methyl pyridone derivative is obtained simply and has a high yield. The simple and effective method has the advantages that there is only a reaction step; a flow is simple; the raw materials and the catalyst are cheap and easily available chemical materials; and a reaction organic solvent can be recovered easily and be recycled so that a reaction process is clean and efficient and large-scale industrial production can be realized.
Owner:HENAN NORMAL UNIV
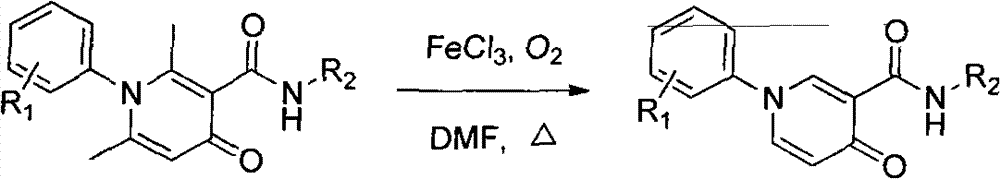
Method for simply and effectively removing 2-position methyl and 6-position methyl of 2, 6-dimethyl-4-pyridinone derivative
The invention provides a method for simply and effectively removing 2-position methyl and 6-position methyl of a 2, 6-dimethyl-4-pyridinone derivative. The method comprises the step of removing two methyl groups in Alpha-position of the 2, 6-dimethyl-4-pyridinone derivative under a heating condition by adopting oxygen as an oxidant and ferric chloride anhydrous as a catalyst. The 2, 6-dedimethylation-pyridinone derivative can be simply obtained along with high yield by the processes of cooling, extracting and separating after the reaction. According to the method, the reaction is just carried out in one step, so that the process is simple; the raw materials and the catalyst are the chemistry and chemical raw materials which are low in price and easy to obtain; and the organic solvent in reaction is easily recovered and recycled, so that the whole reaction is a clean and efficient technological process, and the benefit is provided for massive industrial production.
Owner:HENAN NORMAL UNIV
Chlorination synthesis process of chlorination workshop section in production of pyridinium salt
The invention discloses a chlorination synthesis process of a chlorination workshop section in production of a pyridinium salt. The process provided by the invention adopts 1,2-dichloroethane insteadof conventional dichloromethane as a solvent, a dipole moment of the 1,2-dichloroethane is smaller than that of the dichloromethane, and polarity of 4-chloro-3-methoxy-2-methylpyridine is smaller thanthat of 3-methoxy-2-methyl-4-pyridone, so that improvement of a yield of the 4-chloro-3-methoxy-2-methylpyridine is facilitated; water solubility of the 1,2-dichloroethane is smaller than that of thedichloromethane, so that recycling of the dichloroethane is facilitated, and pollution to the environment is reduced; a binary azeotrope formed by the dichloromethane and water has a boiling point of38.1 DEG C and a water content of 1.5%, and a binary azeotrope formed by the1,2-dichloroethane and water has a boiling point of 71.9 DEG C and a water content of 8.1%, so that when distillation is performed, more water can be carried away, and a use amount of a desiccant can be reduced; and a use amount of phosphorus oxychloride is greatly reduced, and the process does not need vacuum recovery and reuse, thereby reducing safety risk of a reaction.
Owner:ANHUI JINGHE IND
Method for preparing block cationic water-borne polyurethane iron (III) chelates
ActiveCN104945564AInhibit side effectsMild reaction conditionsGlycidyl methacrylateN dimethylformamide
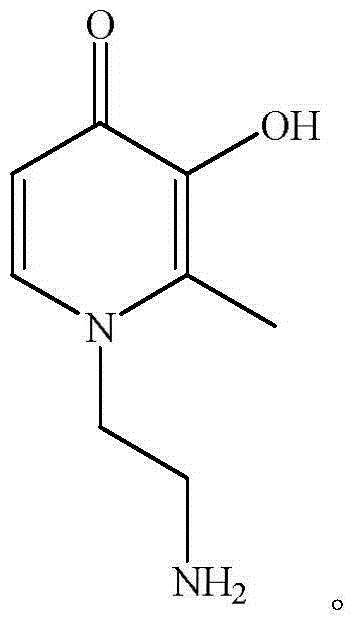
The invention discloses a method for preparing block cationic water-borne polyurethane iron (III) chelates. The method comprises reacting diisocyanate with macromolecular dibasic alcohol, micromolecular dibasic alcohol and a hydrophilic chain extender, adding an end-capping reagent to obtain double bond end-capped polyurethane prepolymer, adding glycidyl methacrylate to carry out the copolymerization reaction, adding amino-containing 3-hydroxyl-4-pyridone derivatives to carry out the ring-opening reaction with epoxy groups, and performing covalent linkage to the two ends of polyurethane to obtain the block cationic water-borne polyurethane iron (III) chelates. The method avoids the side reaction between 3 hydroxyl groups and isocyanate, the reaction conditions are mild, the aftertreatment is simple, the obtained micromolecular chelates are located on side chains at the two ends of a chain of polyurethane, the content is adjustable (0.1-15wt%); and the obtained macromolecular chelates can be dissolved in water, acetone, butanone, N,N-dimethylformamide and dimethylsulfoxide.
Owner:UNIV OF SCI & TECH OF CHINA
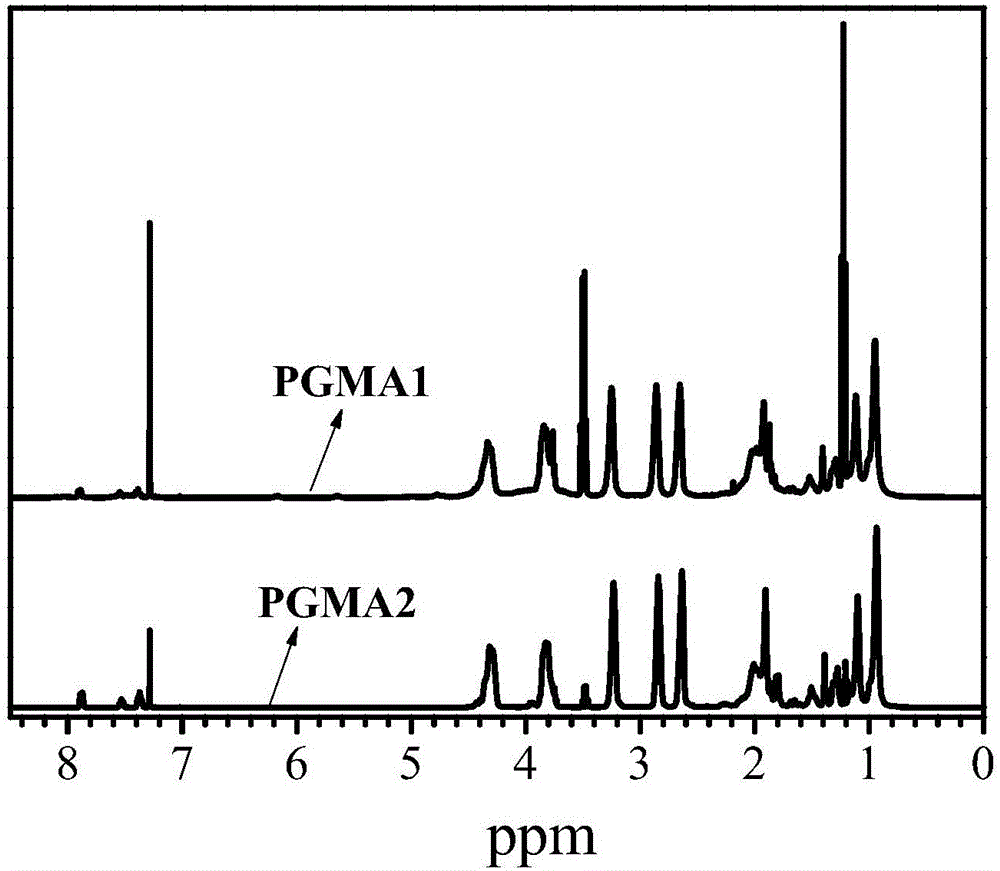
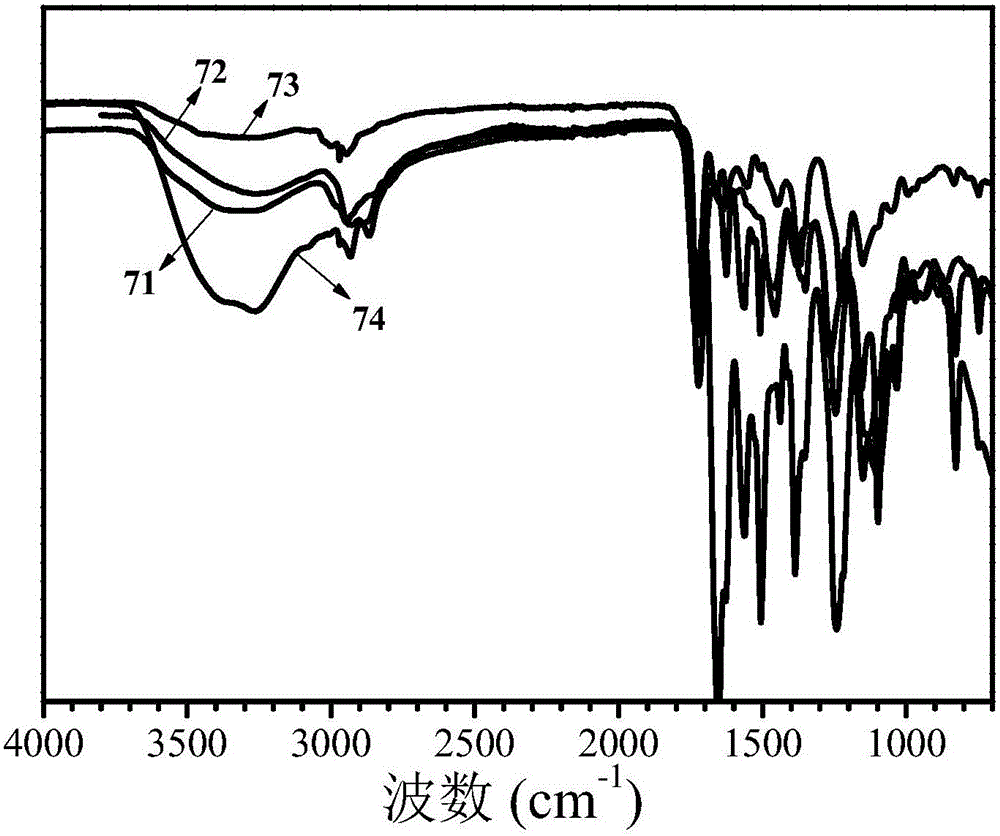
High-molecular iron (III) chelating agent based on 3-hydroxyl-4-pyridinone compounds and preparation method thereof
ActiveCN105037595AAvoid other side effectsMild reaction conditionsWater/sewage treatment by neutralisationWater/sewage treatment by flocculation/precipitationMethylating AgentGlycidyl methacrylate
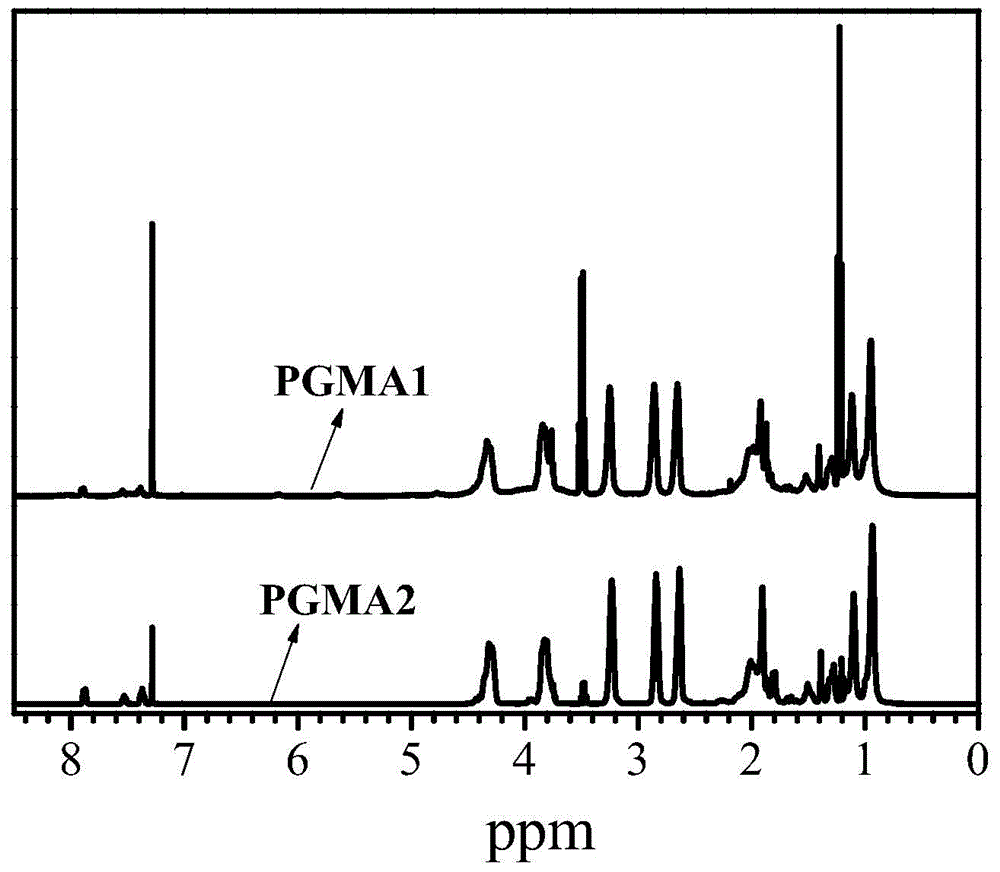
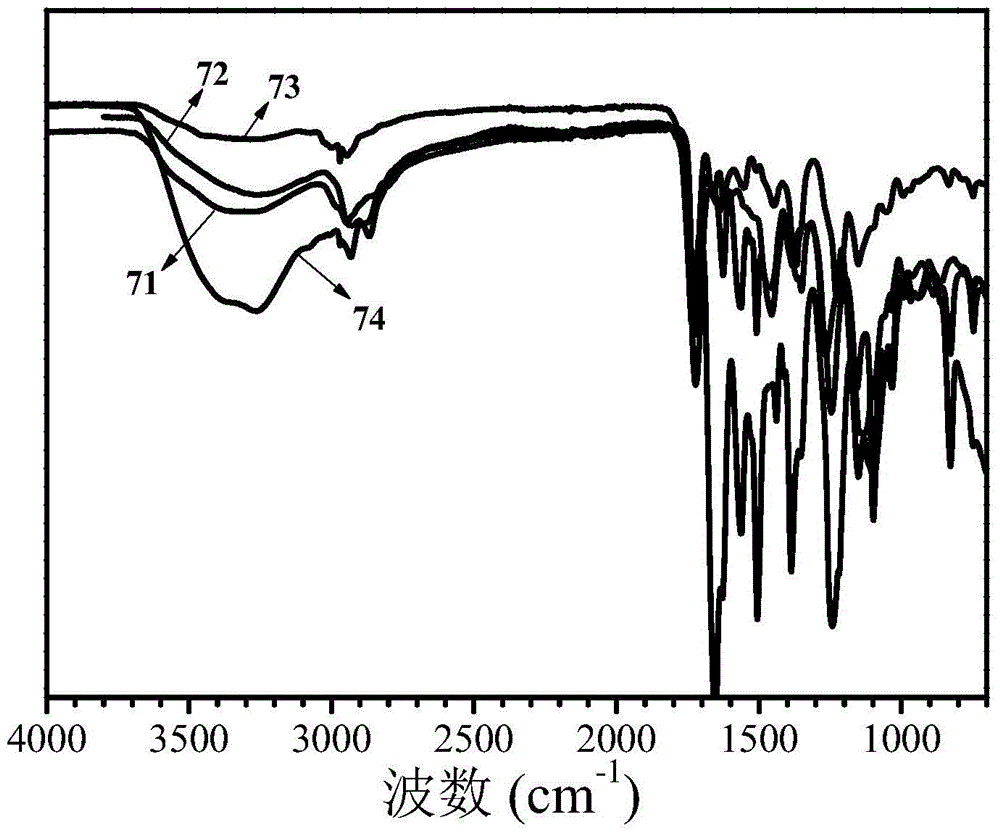
The invention discloses a high-molecular iron (III) chelating agent based on 3-hydroxyl-4-pyridinone compounds and a preparation method thereof. The method comprises the following steps: subjecting epoxy-group-containing glycidyl methacrylate to free radical polymerization so as to obtain poly(glycidyl methacrylate), then adding amine-group-containing 3-hydroxyl-4-pyridinone compounds and then carrying out ring-opening reaction with epoxy groups, and carrying out covalent attachment to a side chain of poly(glycidyl methacrylate) so as to obtain the high-molecular iron (III) chelating agent. The preparation method provided by the invention avoids other side reaction and has the advantages of mild reaction conditions, safe production, simple process, convenient purification, high yield (the maximum being 100%) and facilitation to industrial production. Meanwhile, the problems like insolubility, infusibility, small iron (III) chelate capacity of a conventional high-molecular iron (III) chelating agent are solved, wherein the maximum of the chelating capacity of a ferric ion can reach to 1196 [mu]mol / g.
Owner:UNIV OF SCI & TECH OF CHINA
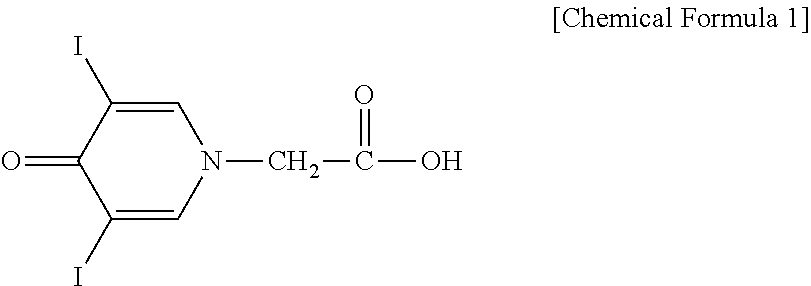
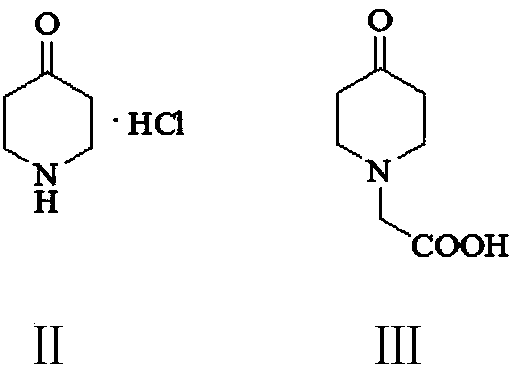
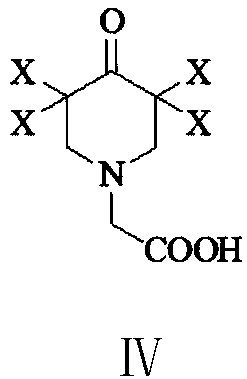
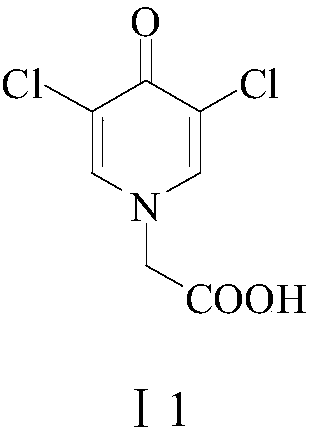
Preparation method of 3,5-dihalogenated-4-pyridone-1-acetic acid
The invention provides a preparation method of 3, 5-dihalogenated-4-pyridone-1-acetic acid, and particularly relates to a preparation method of 3,5-dichloro-4-pyridone-1-acetic acid. The method comprises the following steps: performing a substitution reaction on piperidine-4-ketone hydrochloride (II) with chloroacetic acid in the presence of a proper solvent and an acid-binding agent to obtain 4-piperidone-1-acetic acid (III), carrying out a halogenation reaction on the 4-piperidone-1-acetic acid (III) and a halogenation reagent to obtain 3,3,5,5-tetrahalogenated-4-piperidone-1-acetic acid (IV), carrying out an elimination reaction in the presence of an alkali to remove hydrogen halide, and performing acidification with hydrochloric acid to obtain the 3,5-dihalo-4-pyridinone-1-acetic acid.The method has the advantages of cheap and easily available raw materials, low cost, simple operation, easy realization, less wastewater generation amount, environmental protection, high reaction selectivity, high product yield and purity, and suitableness for industrial production.
Owner:XINFA PHARMA
Inhibitors of catechol O-methyl transferase and their use in the treatment of psychotic disorders
ActiveUS9260413B2Organic chemistryPharmaceutical active ingredientsDiseaseCatechol-O-methyl transferase
Owner:MERCK SHARP & DOHME CORP
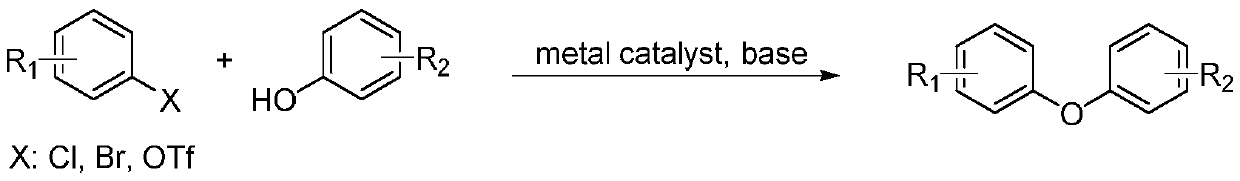
Heterocyclic aromatic ether compounds and synthesis method thereof
InactiveCN106883170BSimple and safe operationEase of industrial productionOrganic chemistryAcetic acidAcetic anhydride
Owner:HENAN POLYTECHNIC UNIV
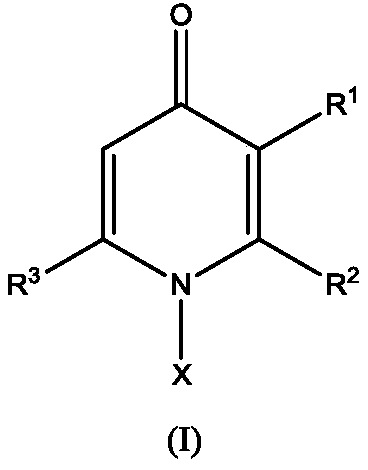
Application of 1-aryl-4-pyridone compound
ActiveCN110622973AExcellent broad-spectrum antifungal activityExtend freshnessBiocideFungicidesDiseaseBacterial disease
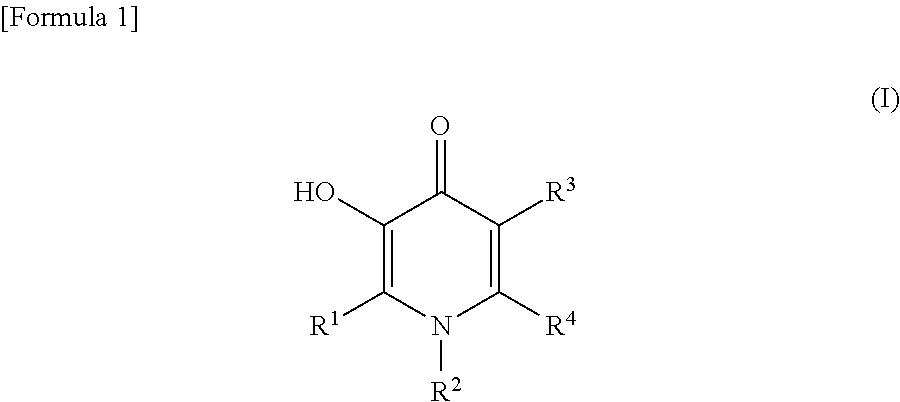
The invention discloses application of a 1-aryl-4-pyridone compound, and provides application of a compound of a structure of a formula (I) shown in the description in inhibiting activity of plant pathogenic bacteria. The compound of the structure of the formula (I) shown in the description has outstanding broad-spectrum antifungal activity upon plant pathogenic bacteria in agricultural production, and meanwhile, has a prevention and treatment effect on bacterial disease of crops. In-vivo biological assay shows that the compound of the structure of the formula (I) shown in the description hasa prevention and control effect greater than 95% on cucumber downy mildew, cucumber target leaf spot, wheat scab and tomato gray mold. Meanwhile, results of postharvest fresh-keeping tests of mangos show that the compound is capable of effectively controlling postharvest diseases of mangos and in addition, prolonging the fresh-keeping time of the mangos. In addition, results show that the compoundis also capable of effectively controlling bacterial leaf blight of rice in pot experiments, and is more effective than a commercial fungicide zhongshengmycin. In conclusion, the 1-aryl-4-pyridone derivative of the formula (I) shown in the description has broad-spectrum plant pathogenic fungus resistance and bacterial activity, and is a type of lead compounds with wide bioactivity.
Owner:HAINAN UNIVERSITY
A kind of polymer iron (iii) chelating agent based on 3-hydroxyl-4-pyridone compound and preparation method thereof
ActiveCN105037595BAvoid other side effectsMild reaction conditionsWater/sewage treatment by neutralisationWater/sewage treatment by flocculation/precipitationGlycidyl methacrylateSide chain
The invention discloses a high-molecular iron (III) chelating agent based on 3-hydroxyl-4-pyridinone compounds and a preparation method thereof. The method comprises the following steps: subjecting epoxy-group-containing glycidyl methacrylate to free radical polymerization so as to obtain poly(glycidyl methacrylate), then adding amine-group-containing 3-hydroxyl-4-pyridinone compounds and then carrying out ring-opening reaction with epoxy groups, and carrying out covalent attachment to a side chain of poly(glycidyl methacrylate) so as to obtain the high-molecular iron (III) chelating agent. The preparation method provided by the invention avoids other side reaction and has the advantages of mild reaction conditions, safe production, simple process, convenient purification, high yield (the maximum being 100%) and facilitation to industrial production. Meanwhile, the problems like insolubility, infusibility, small iron (III) chelate capacity of a conventional high-molecular iron (III) chelating agent are solved, wherein the maximum of the chelating capacity of a ferric ion can reach to 1196 [mu]mol / g.
Owner:UNIV OF SCI & TECH OF CHINA
4-pyridinone compounds and their use for cancer
Disclosed herein is a process for preparing a compound of Formula (I), comprising the steps of: (a) reacting an aniline compound of Formula (II) and an carboxylic acid compound of Formula (III) or an activated carboxylic acid compound thereof, to provide a compound of Formula (IV); and (b) converting said protected amine group attached to said compound of Formula (IV) to an amine group to provide said compound of Formula (I); wherein PAm is a protected amine group. Processes to prepare the compounds of Formulae (II), (III), and (IV) are also disclosed.
Owner:BRISTOL MYERS SQUIBB CO
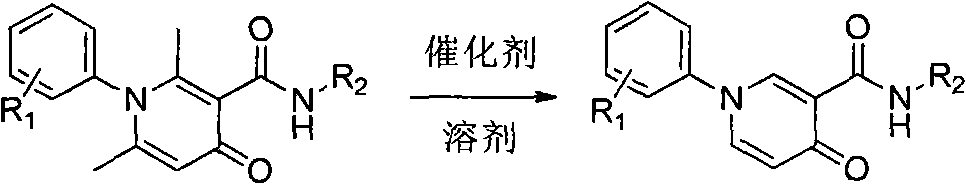
Simple and effective method for high-selectivity removal of alpha-monomethyl of 2,6-dimethyl-4-pyridone derivative
The invention discloses a simple and effective method for high-selectivity removal of an alpha-monomethyl of a 2,6-dimethyl-4-pyridone derivative. The simple and effective method comprises that an alpha-monomethyl of a 2,6-dimethyl-4-pyridone derivative is removed by oxygen as an oxidizing agent in the presence of anhydrous ferric chloride as a catalyst with heating; and after the reaction, cooling, extraction and separation processes are carried out so that a 6-methyl pyridone derivative is obtained simply and has a high yield. The simple and effective method has the advantages that there is only a reaction step; a flow is simple; the raw materials and the catalyst are cheap and easily available chemical materials; and a reaction organic solvent can be recovered easily and be recycled so that a reaction process is clean and efficient and large-scale industrial production can be realized.
Owner:HENAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com