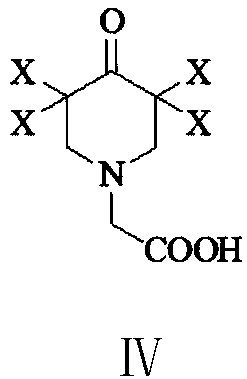
Preparation method of 3,5-dihalogenated-4-pyridone-1-acetic acid
A technology of pyridone and dihalogenation, which is applied in the field of preparation of 3,5-dihalogeno-4-pyridone-1-acetic acid, and can solve the problem of high price of 4-aminopyridine, poor stability of diazonium salt, and operational requirements Advanced problems, to achieve the effect of high target reaction selectivity, good stability and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
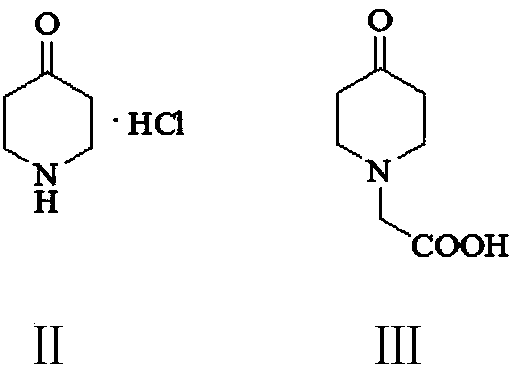
[0050] Example 1: Preparation of 4-piperidone-1-acetic acid (III)
[0051] To the 500-milliliter four-necked flask connected with stirring, thermometer, reflux condenser and constant-pressure dropping funnel, add 60 g of water, 34.5 g (0.25 mol) of potassium carbonate, 21.8 g (0.23 mol) of chloroacetic acid, heat, keep Between 60-65°C, a solution of 27.1 g (0.2 mol) piperidin-4-one hydrochloride and 60 g of water was added dropwise, and the dropwise addition was completed in about 2 hours, after which the reaction was stirred at 70-75°C for 4 hours, and cooled to 20-25°C, 30wt% hydrochloric acid acidification system with pH value of 2.5-3.0, filtered and dried to obtain 28.95 g of white solid 4-piperidone-1-acetic acid with a yield of 92.2% and a liquid phase purity of 99.3%.
Embodiment 2
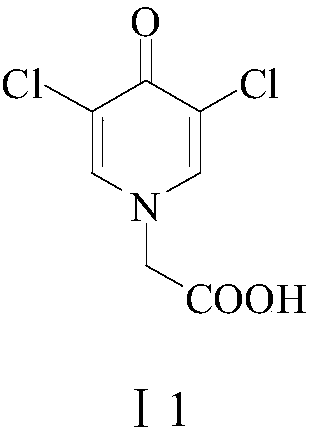
[0052] Example 2: Preparation of 3,5-dichloro-4-pyridone-1-acetic acid (I1)
[0053] To the 500-milliliter four-necked flask connected with stirring, thermometer, reflux condenser and constant pressure dropping funnel, add 100 grams of water, 15.7 grams (0.1 moles) of 4-piperidone-1- Acetic acid, 47.0 g (0.45 mol) 35wt% hydrochloric acid, 52.0 (0.46 mol) 30wt% hydrogen peroxide was added dropwise at 50-55°C for about 3 hours, after that, the reaction was stirred at 55-60°C for 5 hours, cooled to 20-25 ℃, add 35.0 grams (0.35 moles) of 40wt% sodium hydroxide aqueous solution, 0.5 grams of activated carbon, stir at 50-55 ℃ for 2 hours, filter, wash the filter cake with 20 grams of water, combine the filtrates, cool to 20-25 ℃, 30wt % The pH value of the acidification system with hydrochloric acid was 1.5-2.0, filtered and dried to obtain 20.09 g of white solid 3,5-dichloro-4-pyridone-1-acetic acid (I1) with a yield of 90.5% and a liquid phase purity of 99.6%.
[0054] The NMR d...
Embodiment 3
[0056] Example 3: Preparation of 3,5-dichloro-4-pyridone-1-acetic acid (I1) (one-pot method)
[0057] To the 500-milliliter four-necked flask connected with stirring, thermometer, reflux condenser and constant-pressure dropping funnel, add 60 grams of water, 8.8 grams (0.22 moles) of sodium hydroxide, 10.5 grams (0.11 moles) of chloroacetic acid, heat, Keep between 60-65 ℃, add dropwise a solution of 13.6 g (0.1 mol) piperidin-4-one hydrochloride (II) and 30 g of water, the dropwise addition is completed in about 2 hours, and then the reaction is stirred at 70-75 ℃ for 4 hour, cooled to 20-25 ℃, added 52.0 g (0.5 mol) of 35wt% hydrochloric acid, added dropwise 56.7 (0.5 mol) of 30 wt% hydrogen peroxide at 50-55 ℃, dripped in about 3 hours, after that, stirred the reaction at 55-60 ℃ 5 hours, cooled to 20-25 ° C, added 38.0 g (0.38 mol) 40wt% sodium hydroxide aqueous solution, 0.5 g activated carbon, stirred at 50-55 ° C for 2 hours, filtered, washed the filter cake with 20 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com