Application of 1-aryl-4-pyridone compound
A compound and aryl technology, applied in the application field of 1-aryl-4-pyridone compounds, can solve problems such as agricultural disease prevention and control obstacles, and achieve the effect of prolonging the preservation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
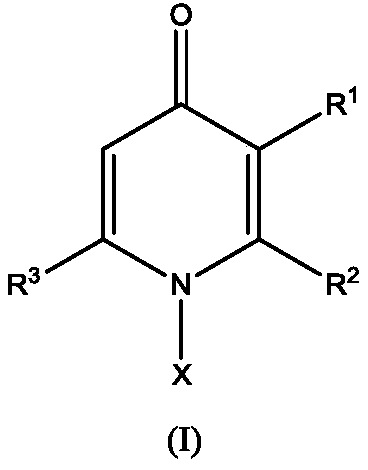
[0038] The present invention also provides a preparation method of the structural compound shown in formula (I), comprising:
[0039] The pyrone of the structure of formula (II) and the amine compound of the structure of formula (III) are heated and refluxed in water to obtain the compound of structure shown in formula (I); or
[0040] The pyrone with the structure of formula (II) and the amine compound with the structure of formula (III) are reacted under high pressure and heated in a mixed solution of water, ethanol and hydrochloric acid to obtain the compound with the structure shown in formula (I);
[0041]
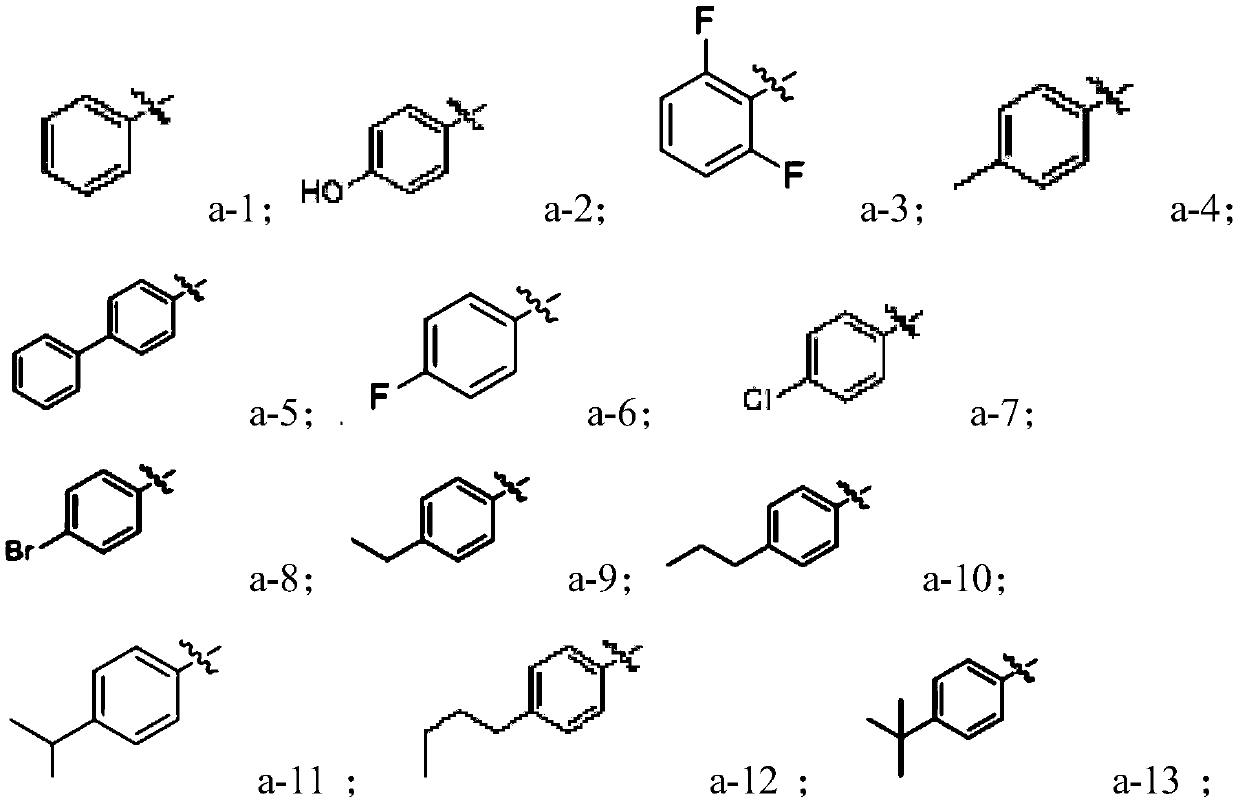
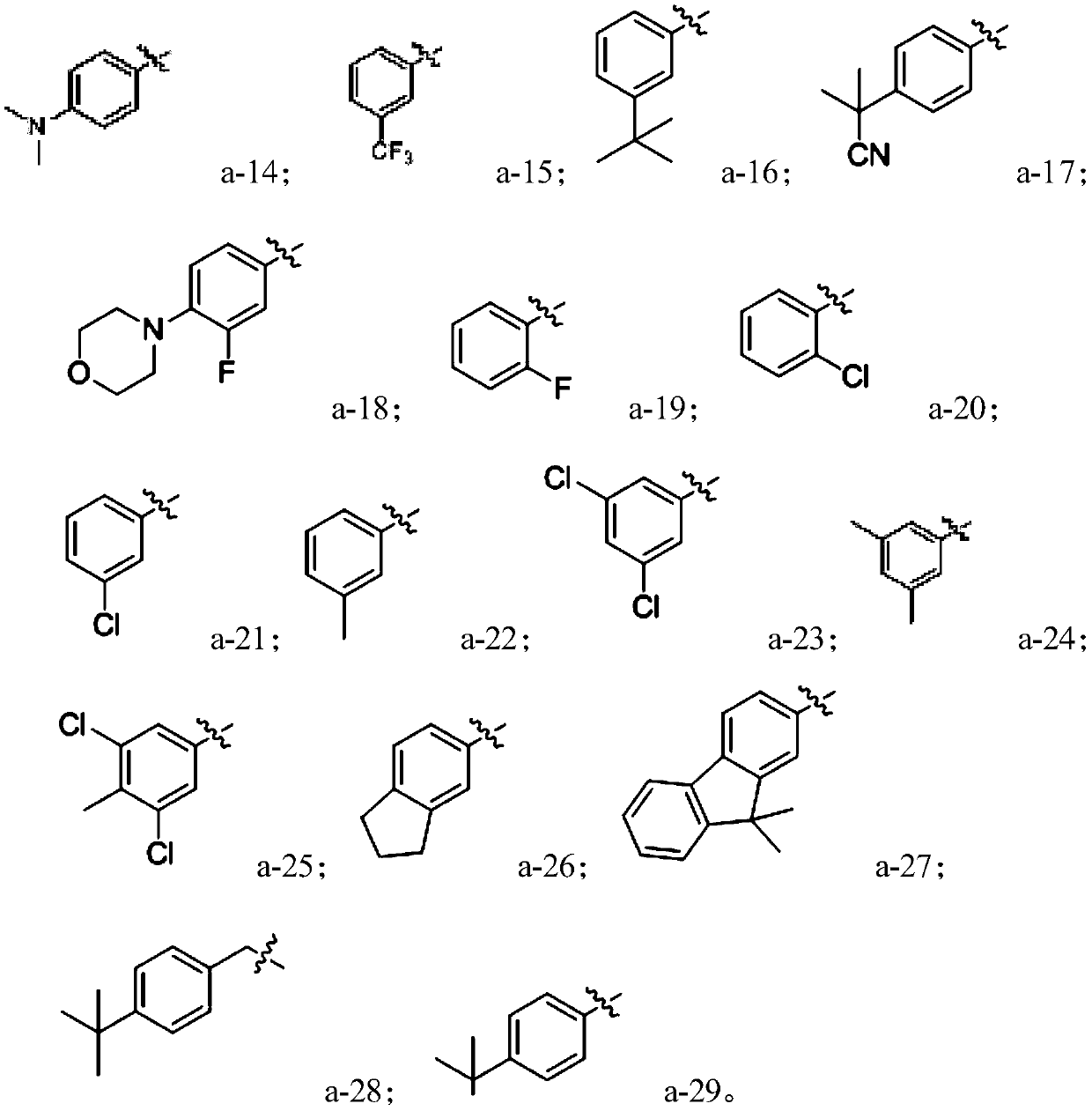
[0042] In the formula, R 1 Alkoxy selected from H, hydroxyl or C1~C10; R 2 is C1~C10 alkyl; R 3 Selected from H, C1-C10 alkyl; X is selected from H, C1-C10 alkyl, C6-C30 aryl, C6-C30 heterocyclic group.
[0043]One of the preparation methods of the compound with the structure shown in formula (I) provided by the present invention preferably can be: heating and r...
Embodiment 1
[0071] Example 1 Preparation of Target Compounds 5 and 6.
[0072] Maltol (10 mmol) was dissolved in 15 mL of water, and excess ammonia (6 mL) was added. The reaction mixture was refluxed for 10 hours, ammonia and solvent were removed under reduced pressure, and the mixture was cooled to room temperature overnight. The solid product obtained is recrystallized from a 50% water / methanol mixture in the presence of activated carbon. Activated carbon was filtered off, and the product was concentrated under reduced pressure. The final product is dried over phosphorus pentoxide in a desiccator. The target compound 5 (1.01 g, 7.93 mmol) was obtained. Gray solid, mp 285-287°C, yield 79%. 1 H NMR (600MHz, DMSO-d 6 )δ8.01(d, J=5.5Hz, 1H, pyridinone), 6.33(d, J=5.5Hz, 1H, pyridinone), 2.23(s, 3H, CH 3 ). 13 C NMR (150MHz, DMSO-d 6 )δ172.6, 154.6, 149.2, 143.0, 113.6, 14.0.ESI-HRMS: m / z[M+H] + calcd.for[C 6 h 8 NO 2 ]: 126.1243; found: 126.1238.
[0073] The synthesis of targe...
Embodiment 2
[0075] Example 2: Preparation of target compound 7-37.
[0076] Maltol (10 mmol) and excess aniline (15 mmol) were added to an acidic solution of 18.0 mL of water, 0.4 mL of HCl and 2.0 mL of ethanol (pH=5). The resulting mixture was heated in an autoclave at 160° C. for 12 hours. After completion of the reaction, the reaction mixture was adjusted to pH = 7 using sodium hydroxide solution (2N), and the product was collected by filtration. Finally, by silica gel column chromatography (CH 2 Cl 2 :CH 3 OH=50:1) to purify the residue to obtain the target compound 7 (1.67g, 8.3mmol). White solid, mp 206-207°C, yield 83%. 1 HNMR (500MHz, Chloroform-d) δ7.53 (m, J = 4.8, 1.8Hz, 3H, Ph), 7.30 (d, J = 7.3Hz, 1H, pyridinone), 7.29–7.26 (m, 2H, Ph) ,6.46(d,J=7.3Hz,1H,pyridinone),2.10(s,3H,CH 3 ). 13C NMR (125MHz, Chloroform-d) δ170.3, 145.8, 141.9, 137.6, 130.0, 129.7, 128.5, 126.9, 111.0, 13.8. ESI-HRMS: m / z[M+H] + calcd.for[C 12 h 12 NO 2 ]: 202.0863; found: 202.0858.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com