A kind of 1-trifluoromethyl cinnamyl alcohol derivative and its preparation method and application
A technology of trifluoromethyl and cinnamyl alcohol, applied in the field of pesticides, can solve the problems of less research on fungicides, and achieve the effects of broad-spectrum anti-phytopathogenic fungi and bacteria activity and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
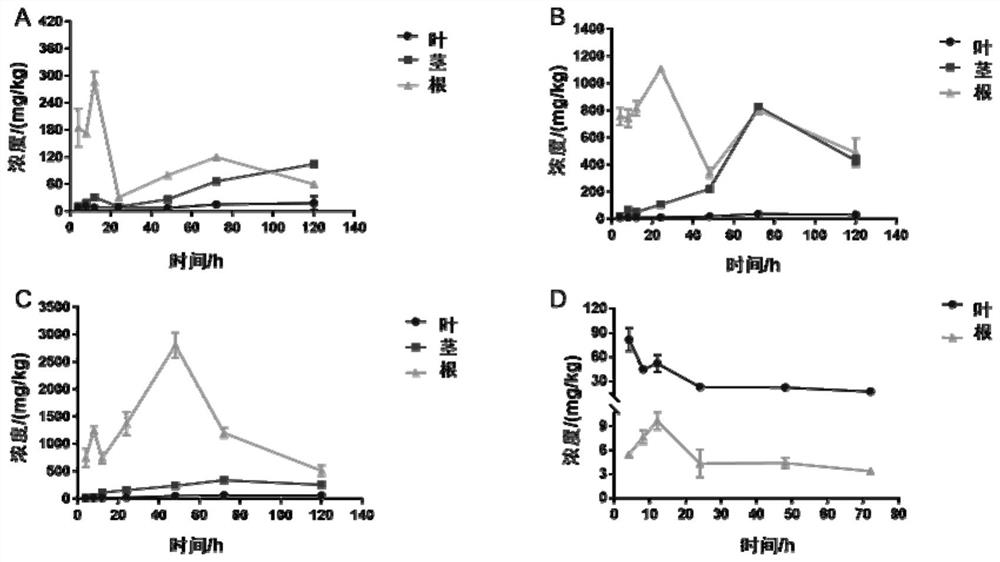
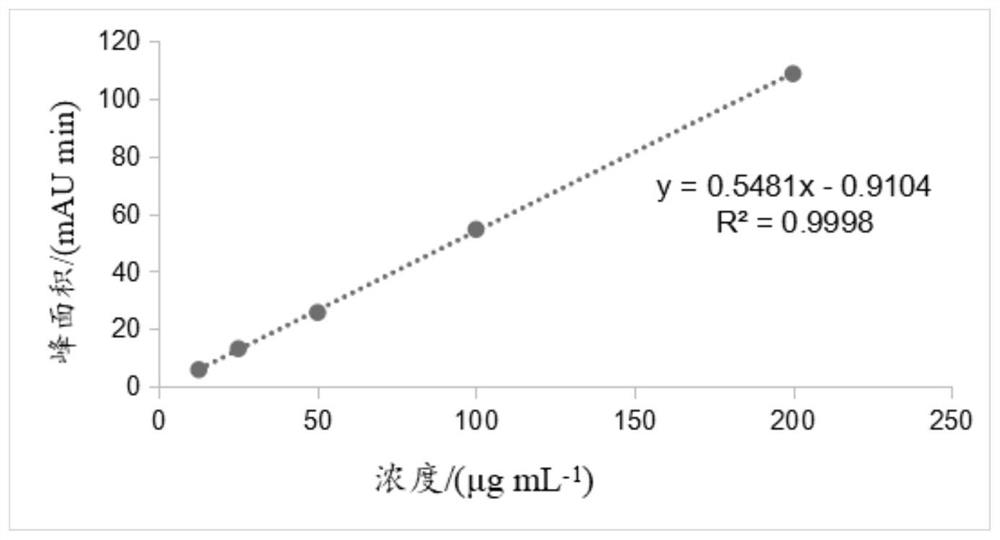
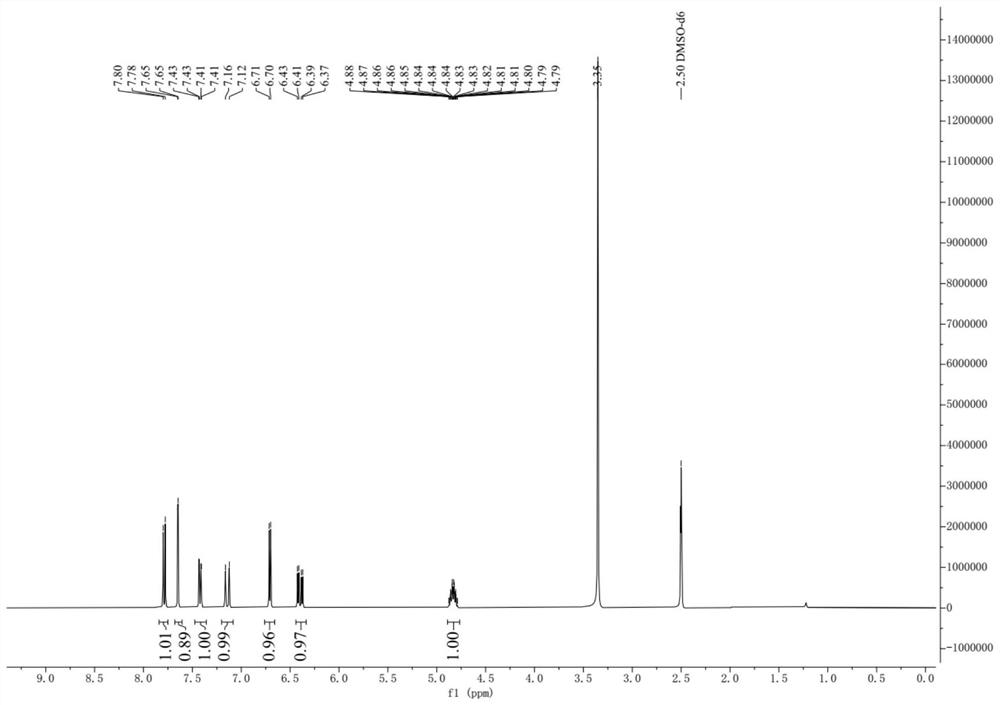
Image
Examples
Embodiment 1
[0084] 1-Trifluoromethyl cinnamyl alcohol derivatives, whose structural formulas are shown in (I-1) to (I-4).
[0085] The preparation method of the 1-trifluoromethyl cinnamyl alcohol derivative comprises the following steps: under nitrogen conditions, 1 mmol of isopropyl titanate (Ti(O-i-Pr) 4 ) was slowly added to 1 mmol of 3-bromo-1,1,1-trifluoroacetone, 1 mmol of benzaldehyde derivative and 1 mmol of triphenylphosphine (Ph 3 P) in the mixture. After stirring the reaction at 80°C for 24 hours, the reaction product was obtained; the reaction product was treated with 5% HCl (10ml), and then extracted with ethyl acetate (3×20ml). After the extraction, the organic layer was washed with water (3×10ml). Add anhydrous sodium sulfate to dry, and evaporate the solvent to obtain a crude product; the crude product is separated and eluted by a chromatographic column to obtain the target compound; the eluent of the chromatographic column is petroleum ether and ethyl acetate at 20:1 Mi...
Embodiment 2
[0097] 1-Trifluoromethyl cinnamyl alcohol derivatives, whose structural formulas are shown in (I-5) to (I-12).
[0098] The preparation method of the 1-trifluoromethyl cinnamyl alcohol derivative includes the following steps: adding 1 mmol of 1-trifluoromethyl cinnamaldehyde derivative and 1.2 mmol of Grignard reagent to 5 mL of tetrahydrofuran (THF) at room temperature , stirred, heated to 66°C under reflux for 2 h, then the reaction was cooled to room temperature, added to 2M HCl (15 mL) and extracted with ethyl acetate (3 x 20 mL). The combined organic layers were washed with water (3 x 10 ml), dried over anhydrous sodium sulfate, and the solvent was removed in vacuo to give the crude product. The crude product is separated and eluted by a chromatographic column to obtain the product; the eluent of the chromatographic column is obtained by mixing petroleum ether and ethyl acetate in a volume ratio of 20:1.
[0099] The reaction formula of this reaction is as follows:
[0...
Embodiment 3
[0119] A 1-trifluoromethyl cinnamyl alcohol derivative, the structure of which is shown in (I-13):
[0120] The preparation method of the 1-trifluoromethyl cinnamyl alcohol derivative includes the following steps: dissolving 1 mmol of 1-trifluoromethyl cinnamaldehyde derivative and 1.2 mmol of trifluoromethyl trimethyl silane in ethylene glycol in methyl ether (5 mL). 0.02 mmol of cesium fluoride was added at 0 °C, and the reaction was carried out at room temperature for 4 h. Then, 1.2 mmol of tetrabutylammonium fluoride and 5M HCl (5 mL) were added, and the reaction was continued for 2 h at room temperature to obtain the reaction product. The reaction product was treated with water (10 mL) and extracted with ethyl acetate (3 x 20 mL). The organic layer was washed with water (3×10 mL), dried and evaporated to remove the solvent to obtain a crude product; the crude product was separated and eluted by a chromatographic column to obtain the target compound; the eluents of the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com