Antifungal compound and application thereof
A compound and antifungal technology, applied in the field of compounds, can solve the problems of poor patient tolerance, many adverse reactions, long half-life, etc., and achieve the effect of excellent broad-spectrum antifungal activity and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
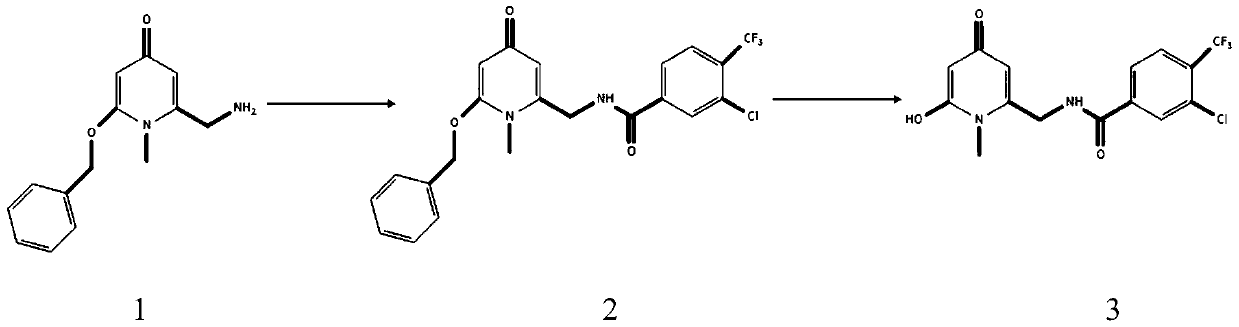
[0017] Embodiment 1 prepares compound
[0018]
[0019] Put compound 1 (500mg) in a 50mL dry two-necked bottle, under nitrogen protection, add 10mL of anhydrous DMF, drop 1mL of anhydrous pyridine at 0°C at 1mL / min, and then dropwise add 3-chloro -4-trifluoromethyl-benzoyl (400mg), react at room temperature for 2-4 hours, add water to quench the reaction, extract with dichloromethane (20×3), and wash the organic phase 3 times with saturated brine, without Dry over sodium sulfate, filter, collect the filtrate and concentrate under reduced pressure, perform column chromatography, the eluent is chloroform:methanol (V:V) = 30:1, and compound 2 is obtained as a colorless viscous liquid.
[0020] Compound 2 (250 mg) was placed in a single-port reaction flask, dissolved in 50 mL of methanol, 100 mg of 10% Pd / C was added, vacuumed, and the reaction system was reacted overnight at room temperature under the protection of hydrogen; after the reaction was completed, the reaction solut...
Embodiment 2
[0024] Embodiment 2 (antifungal activity test)
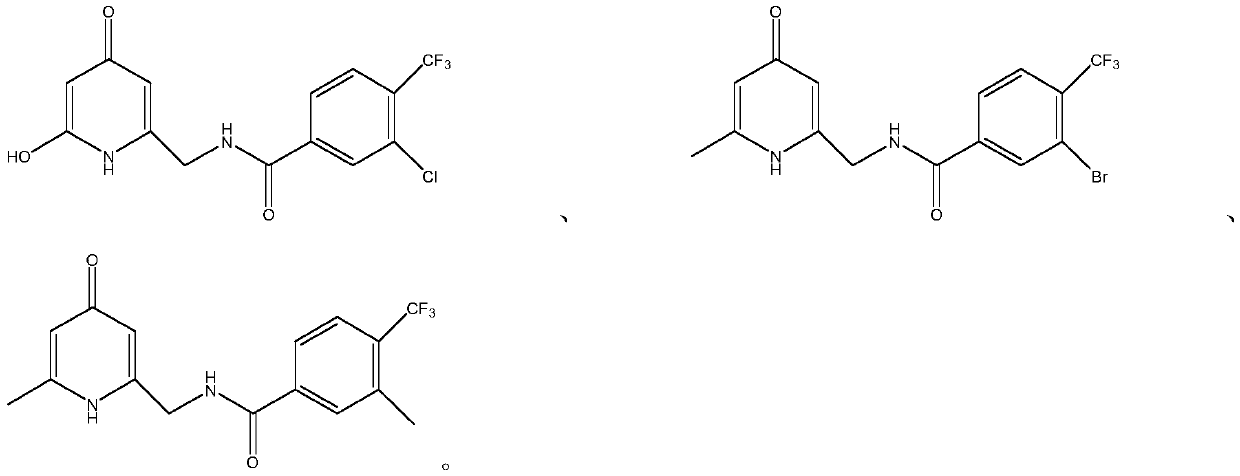
[0025] 1. Experimental strain: Candida albicans (CMCC98001).
[0026] 2. Culture medium: Sabouraud medium was used for Candida albicans.
[0027] The specific formula of the medium is as follows: Sabouraud medium: peptone 10g, glucose 40g, add water to make 1 liter solution.
[0028] 3. Preparation of bacterial suspension:
[0029] The strains are stored on a slant semi-solid medium to maintain the purification and activity of the colony (preserved in a refrigerator at 4°C). When in use, the colonies are inoculated in the liquid medium, cultured on a shaker at 27°C for 24-48 hours, and the concentration of the bacterial suspension is controlled between 1-5×108 / ml with a hemocytometer.
[0030] 4. Preparation of antibacterial compounds:
[0031] compound They were numbered as compounds a, b, and c in sequence. Compounds a, b, and c were dissolved in (dimethyl sulfoxide) DMSO, prepared to 10 mg / ml, filtered through a 0.2 μm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com