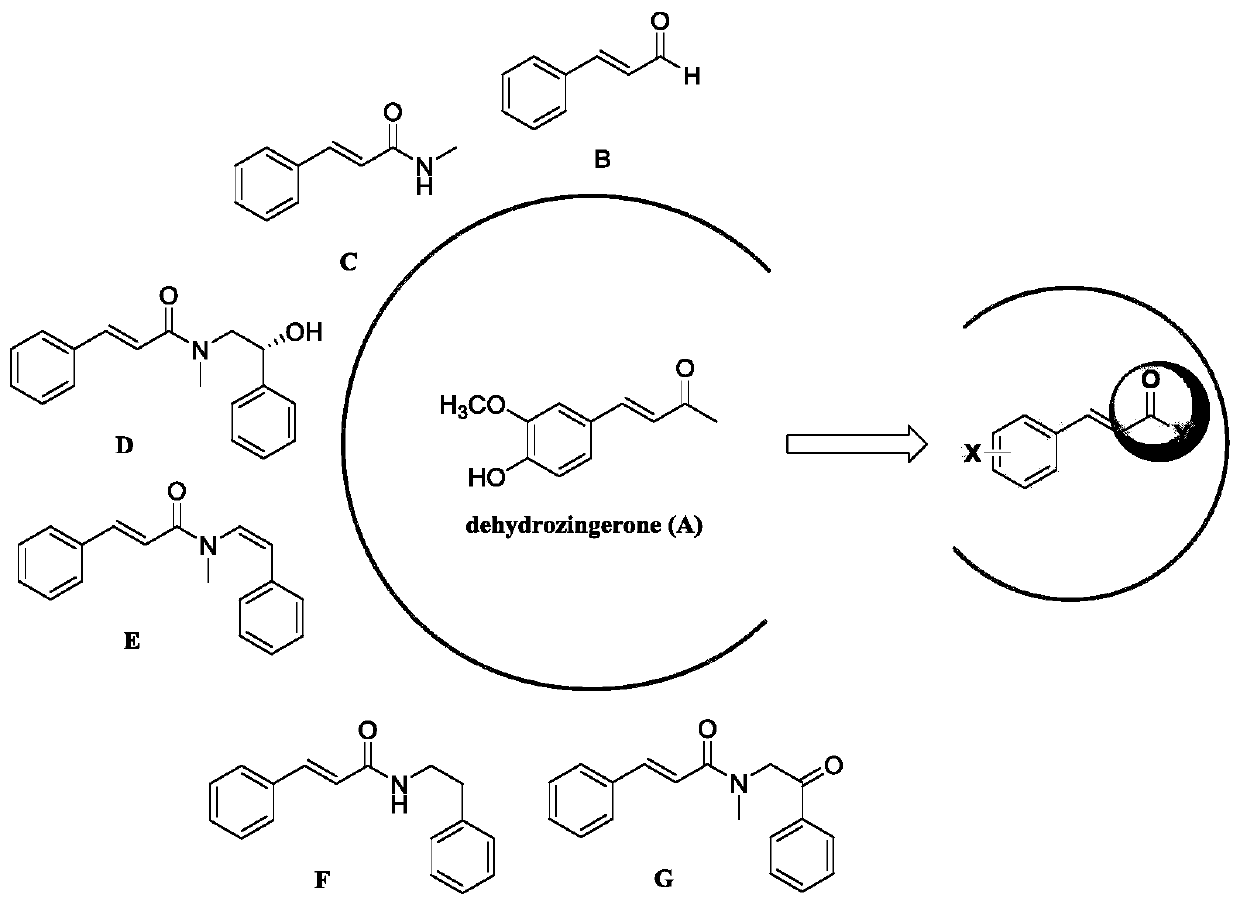
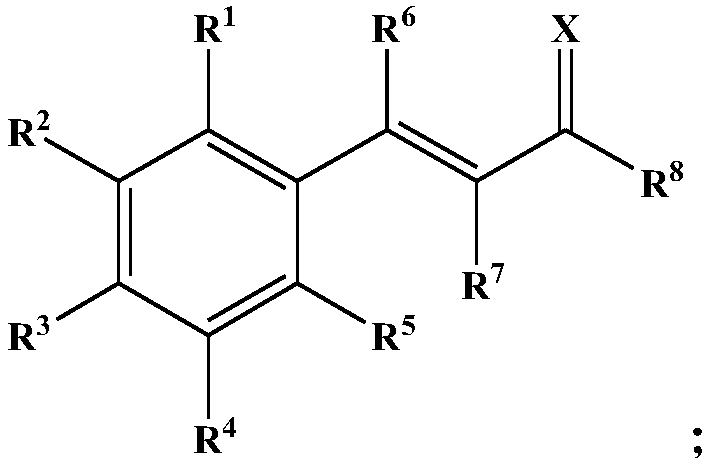
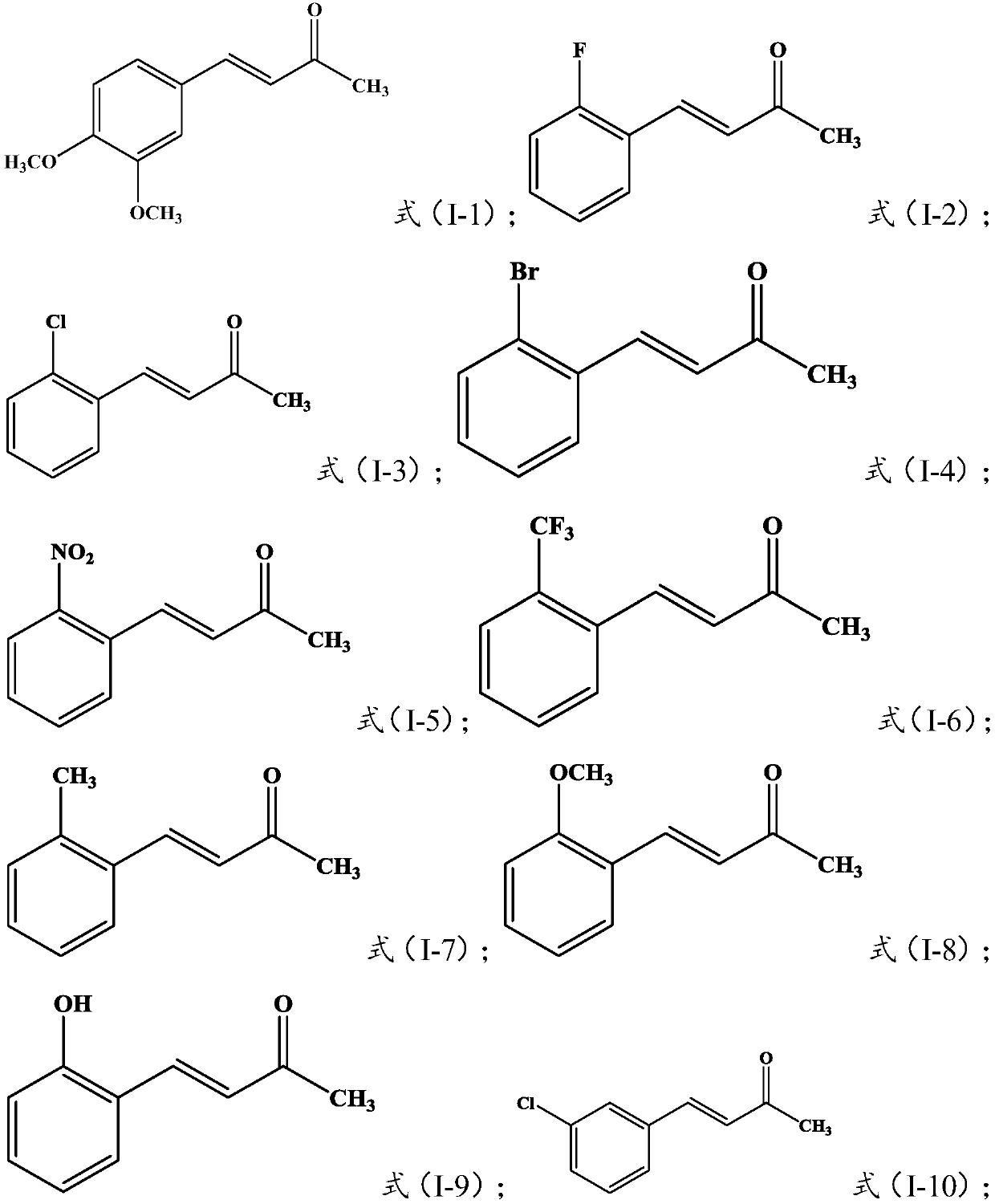
Dehydrozingerone derivative and preparation method and application thereof
A technology of dehydrozingerone and derivatives, applied in the field of pesticides, can solve the problem of less dehydrozingerone and achieve excellent broad-spectrum antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a kind of preparation method of above-mentioned dehydrozingerone derivative, comprising:
[0049] The substituted benzaldehyde shown in formula (1) is reacted with the substituted acetone shown in formula (2) to obtain dehydrozingerone derivatives shown in formula (I).
[0050]
[0051] Among them, R 1 ~R 5 each independently selected from hydrogen, halogen, nitro, alkyl, substituted alkyl, alkoxy or hydroxyl; the substituents in the substituted alkyl are selected from one or more of halogen, nitro and hydroxyl;
[0052] R 8 selected from alkyl, alkoxy or substituted alkyl; the substituent in the substituted alkyl is selected from one or more of halogen, nitro and hydroxyl.
[0053] In the present invention, the R 1 ~R 5 and R 8 All are the same as above, and will not be repeated here.
[0054] The substituted benzaldehyde shown in the formula (1) and the substituted acetone shown in the formula (2) are preferably carried ou...
Embodiment 1
[0084] Embodiment 1: Preparation of Target Compounds I-1~I-24
[0085] Dissolve morpholine (1mmol) in ether (10mL), cool to 0°C in an ice bath, absorb trifluoroacetic acid (1mmol) and dissolve in 5mL ether solution, then slowly add the ether solution of trifluoroacetic acid to morpholine ether In the solution, after incubation for 1 h, return to room temperature, quickly filter off the solvent with a Buchner funnel under reduced pressure, and wash the white solid with diethyl ether (20 mL), repeat 3 times, and use it as a catalyst for the next reaction after drying. Take the prepared catalyst (1mmol), dissolve 3,4-dimethoxybenzaldehyde (5mmol) in 10mL acetone solution, and after reflux reaction for 48h, cool to room temperature, dilute with 20mL ethyl acetate, and wash with saturated NaHCO 3 solution and brine washing (20mL×3), liquid separation, after the organic phase was dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporator under reduced press...
Embodiment 2
[0112] Embodiment 2: the preparation of target compound II-25, II-26
[0113] At 0°C, trimethyl phosphonoacetate (2 mmol) and 2,4-dichlorobenzaldehyde (2.1 mmol) were dissolved in 10 mL of DMF (N,N-dimethylformamide), followed by the slow addition of 28% sodium methoxide dropwise Methanol solution (2mmol), after incubation for 2h, diluted with 20mL distilled water, extracted with ethyl acetate (20mL×3), the organic phase was washed with anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain a crude product, which was separated by column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain a total of 0.45 g (1.93 mmol) of the target compound II-25 as a white solid. The yield was 92%, m.p. 113-115°C. 1 H NMR (500MHz, Chloroform-d) δ8.01 (d, J = 16.0Hz, 1H), 7.55 (d, J = 8.5Hz, 1H), 7.44 (d, J = 2.1Hz, 1H), 7.28–7.25 (m,1H),6.41(d,J=16.0Hz,1H),3.82(s,3H). 13 C NMR (125MHz, Chloroform-d) δ166.7, 139.4, 136.4, 135.5, 131.3, 130.0,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com