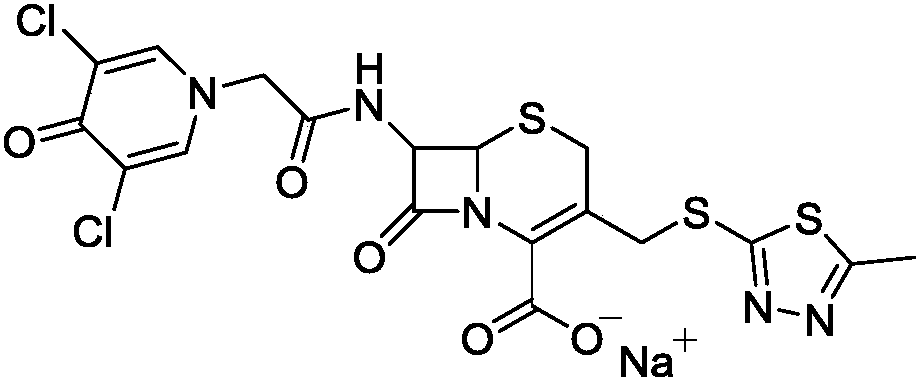
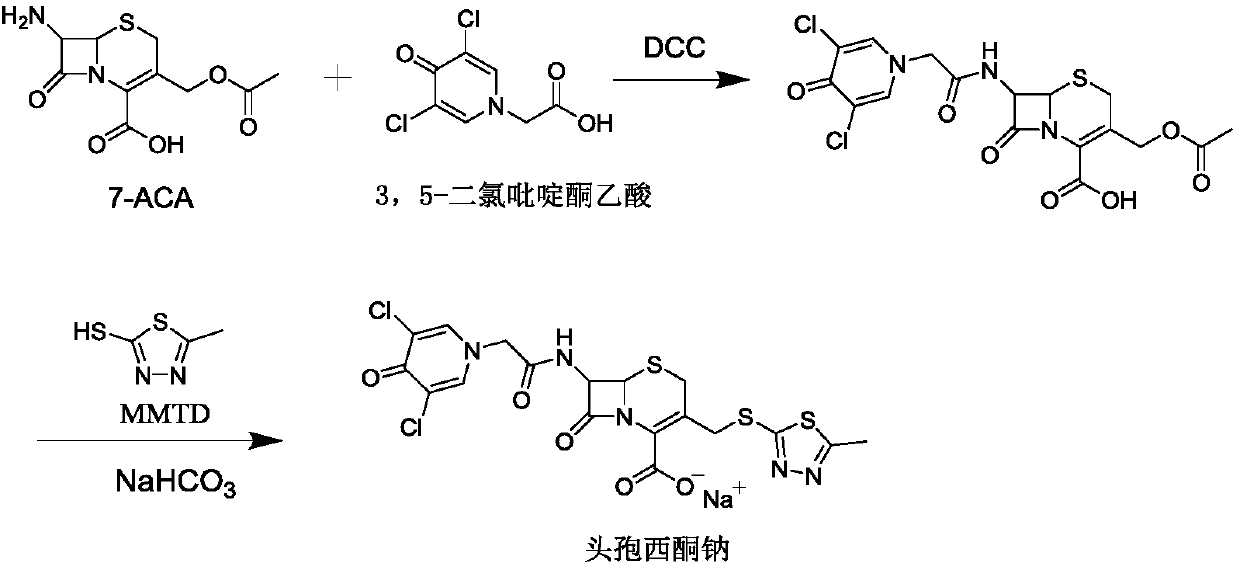
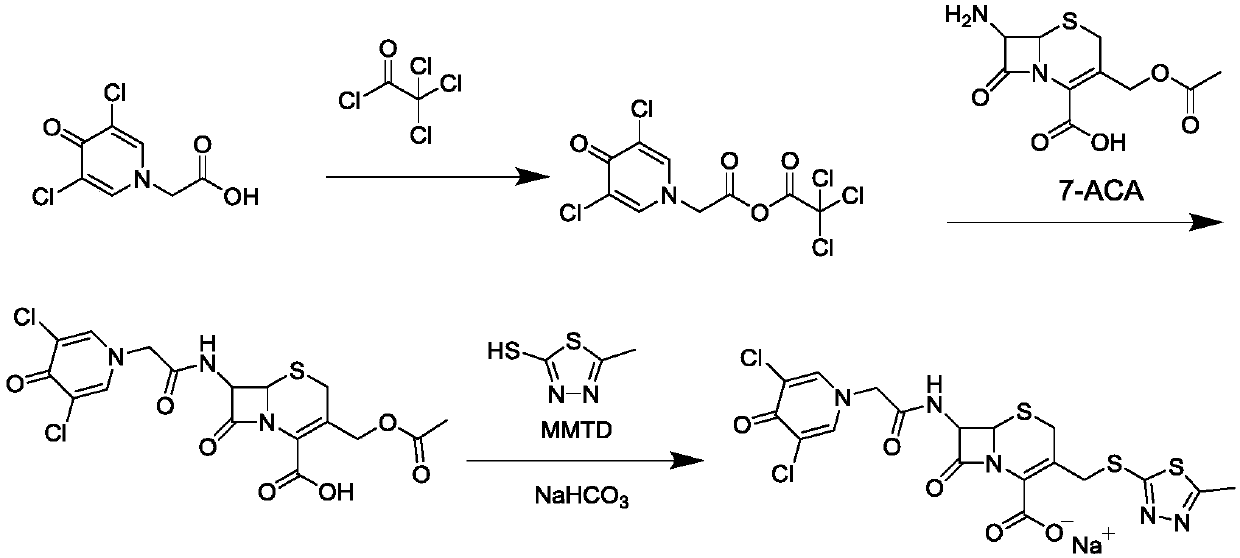
Synthetic method of cefazedone sodium
A technology of cefazedone sodium and its synthesis method, which is applied in the field of drug synthesis, can solve the problems of high reactivity of active esters and difficult control, and achieve the effects of ensuring stability and improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 60mL of dimethyl carbonate and 7.06g of citric acid into a 500mL three-necked flask, add 4.85g of MMTD while stirring, add 10.00g of 7-ACA, and slowly add boron trifluoride-dimethyl carbonate complex 34.82 g, control the temperature at 20-30°C, and monitor the reaction by HPLC after 1 hour, add 1.28g of sodium dithionite, stir for 10 minutes, transfer to water, add 80mL of isopropanol, slowly add concentrated ammonia water to adjust the pH to 3.0, and control the dropping time After 30-60 minutes, cool down to 0-10°C to crystallize for 1 hour. Suction filtration yielded 12.74 g of 7-TDA wet product, with a purity of 99.4% by HPLC and a maximum of 0.10% impurity.
[0036] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of 3,5-dichloropyridoneacetic acid, cool down to 0-10°C, slowly add 5.85g of N,N-diisopropylethylamine dropwise, and stir After 15 minutes, it was raised to room temperature, 33.06 g of dithiodibenzothiazole was added, a...
Embodiment 2
[0039] (1) Add 60mL of dimethyl carbonate and 11.03g of tartaric acid into a 500mL three-necked flask, add 4.85g of MMTD while stirring, add 10.00g of 7-ACA, and slowly add 40.62g of boron trifluoride-dimethyl carbonate complex, Control the temperature at 20-30°C and react. After 1 hour, HPLC monitors the end of the reaction. Add 1.28g of sodium dithionite, stir for 10 minutes, transfer to water, add 80mL of isopropanol, slowly add concentrated ammonia water to adjust the pH to 4.5, and control the dropping time for 30~ After 60 minutes, the temperature was lowered to 0-10°C for 1 hour of crystallization. Suction filtration yielded 12.88 g of 7-TDA wet product, with a purity of 99.3% detected by HPLC and a maximum of 0.09% impurity.
[0040] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of 3,5-dichloropyridoneacetic acid, cool down to 0-10°C, slowly add 3.31g of N,N-dimethylformamide dropwise, and stir for 15min , raised to room temperature, added...
Embodiment 3
[0043] (1) Add 60mL of dimethyl carbonate and 6.62g of acetic acid into a 500mL three-necked flask, add 4.85g of MMTD while stirring, add 10.00g of 7-ACA, and slowly add 46.42g of boron trifluoride-dimethyl carbonate complex, Control the temperature at 20-30°C and react. After 1 hour, HPLC monitors the end of the reaction. Add 1.28g of sodium dithionite, stir for 10 minutes, transfer to water, add 80mL of isopropanol, slowly add concentrated ammonia water to adjust the pH to 4.0, and control the dropping time for 30~ After 60 minutes, the temperature was lowered to 0-10°C for 1 hour of crystallization. Suction filtration yielded 12.80 g of 7-TDA wet product, with a purity of 99.3% by HPLC and a maximum of 0.08% impurity.
[0044] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of 3,5-dichloropyridoneacetic acid, cool down to 0-10°C, slowly add 3.94g of N,N-dimethylacetamide dropwise, and stir for 15min , raised to room temperature, added 33.06 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com