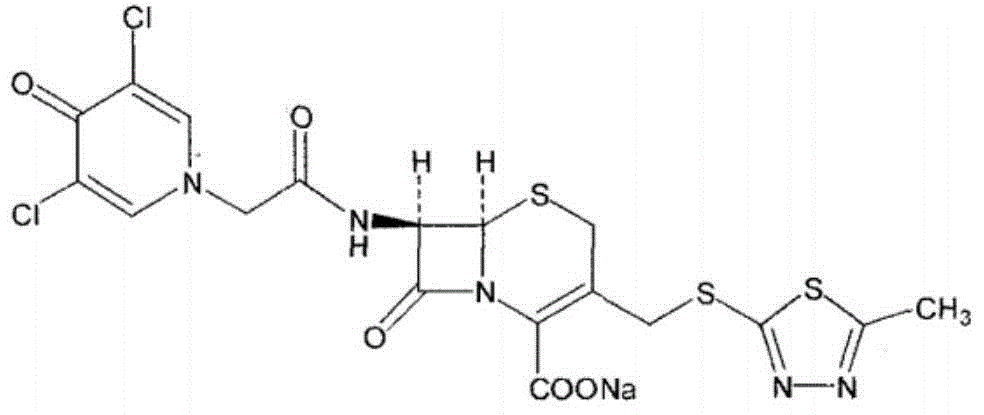
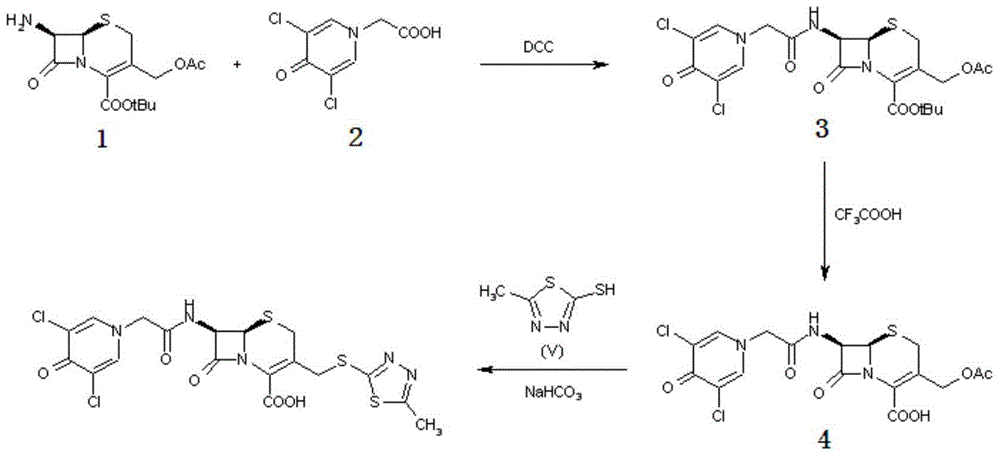
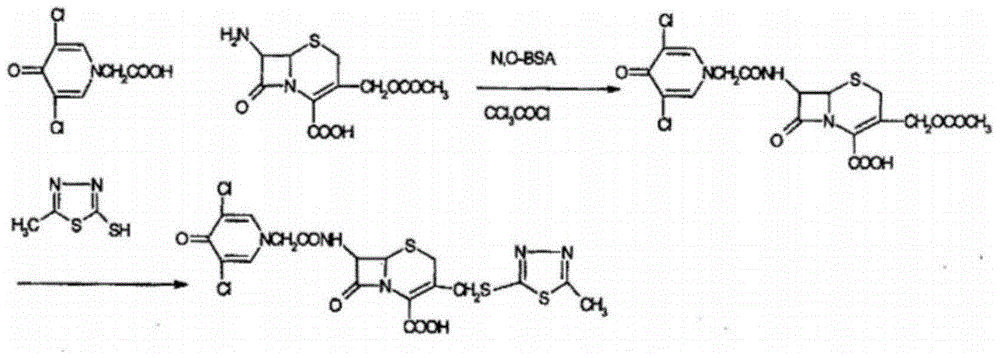
Synthetic process of novel cephalosporin anti-infective drug
A synthesis process and technology for cephalosporins, applied in the field of drug synthesis, can solve problems such as being unsuitable for industrialized large-scale production, high industrial production cost, difficult to find purification methods, etc., and achieve reduction of by-products, product cost reduction, and easy post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of thioester compound (formula III):
[0037] In a dry reaction flask, add 200ml of tetrahydrofuran, 22.2g of 3,5-dichloro-4-pyridone-1-acetic acid (formula I, 0.1mol) and 2-mercapto-5-methyl-1,3, 14.55 g of 4-thiadiazole (formula II, 0.11 mol), stirred, then added 12.2 g (0.12 mol) of triethylamine, stirred at room temperature for 1 hour, then slowly added dropwise the tetrahydrofuran solution in which the catalyst triethyl phosphite was dissolved (Triethyl phosphite 20g dissolved in 50ml of tetrahydrofuran, 0.12mol), drop it off within 1 hour, control the temperature at 20-25°C and stir the reaction for 1 hour, filter, cool the filtrate to below 10°C, precipitate crystals, filter with suction, Vacuum drying below 40°C yielded 32.7 g of thioester compound (Formula III, 0.097 mol), with a yield of 97% and an HPLC purity of 99.5%.
[0038] (2) Preparation of Cefazedone Sodium (Formula IV):
[0039] Under the protection of nitrogen, add 24.5g (0.09mol) of ...
Embodiment 2
[0041] (1) Preparation of thioester compound (formula III):
[0042] In a dry reaction flask, add 180ml of tetrahydrofuran, 22.2g of 3,5-dichloro-4-pyridone-1-acetic acid (formula I, 0.1mol) and 2-mercapto-5-methyl-1,3, 14.55 g of 4-thiadiazole (formula II, 0.11 mol), stirred, then added 6.1 g (0.06 mol) of triethylamine, 0.8 g (0.01 mol) of pyridine, stirred at room temperature for 1 hour, then slowly added dropwise the dissolved catalyst The tetrahydrofuran solution of isopropyl chloroformate (14.7 g of isopropyl chloroformate dissolved in 50 ml of tetrahydrofuran, 0.12 mol), dripped within 1 hour, stirred and reacted at a temperature of 20-25 ° C for 1 hour, filtered, and the filtrate was cooled to 10 Below ℃, crystals were precipitated, filtered by suction, and dried under vacuum below 40℃ to obtain 32.8 g of thioester compound (Formula III, 0.0966 mol), the yield was 96.6%, and the HPLC purity was 99.2%.
[0043] (2) Preparation of Cefazedone Sodium (Formula IV):
[004...
Embodiment 3
[0046] (1) Preparation of thioester compound (formula III):
[0047] In a dry reaction flask, add 200ml of dichloromethane, 33.3g of 3,5-dichloro-4-pyridone-1-acetic acid (formula I, 0.15mol) and 2-mercapto-5-methyl-1, 20.8 g of 3,4-thiadiazole (formula II, 0.1575 mol), stirred, then added 6.1 g (0.06 mol) of triethylamine, 0.95 g (0.012 mol) of pyridine, stirred at room temperature for 1 hour, then slowly added the dissolved Dichloromethane solution with catalyst triethyl phosphite (triethyl phosphite 14.7g dissolved in 50ml of dichloromethane), drop it off within 1 hour, control the temperature at 20-25°C and stir for 1 hour, filter, and cool the filtrate Cool below 10°C to precipitate crystals, filter with suction, and dry under vacuum below 40°C to obtain 49.3 g of thioester compound (Formula III, 0.1452 mol), with a yield of 96.8% and an HPLC purity of 99.0%.
[0048] (2) Preparation of Cefazedone Sodium (Formula IV):
[0049] Under the protection of nitrogen, add 32.7g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com