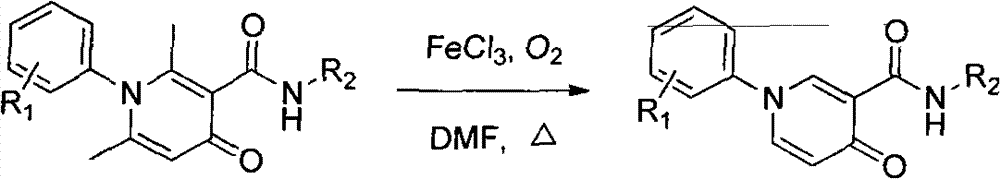
Method for simply and effectively removing 2-position methyl and 6-position methyl of 2, 6-dimethyl-4-pyridinone derivative
A technology of pyridone and derivatives, applied in the field of removal of 2, can solve the problems of high reaction temperature, strong alkali, strong acid, etc., and achieve the effect of low volatility, few experimental steps and high chemical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Add 2,6-dimethyl-4-oxo-N,1-di-p-tolyl-1,4-dihydropyridine-3-carboxamide (346 mg, 1.0 mmol) and anhydrous FeCl to a 25 mL round bottom flask 3 (195mg, 1.2mmol), and 2.0mL DMF, react in an oxygen (1.0atm) atmosphere, the reaction process is monitored by LC-MS, after the reaction, the system is naturally cooled to room temperature, and 20mL of water is added to the reaction system and stirred at room temperature. An insoluble solid precipitated out and was suction filtered, and the insoluble solid was purified by column chromatography to obtain 4-oxo-N,1-di-p-tolyl-1,4-dihydropyridine-3-carboxamide (232 mg, 73%).
[0019] 4-Oxo-N, 1-di-p-tolyl-1, 4-dihydropyridine-3-carboxamide
[0020] Colourless crystal.mp: 195-197℃; Yield: 73%, 1 H NMR (400MHz, CDCl 3 )δ12.33(s, 1H), 8.80(d, J=2.5Hz, 1H), 7.66(dd, J=7.8, 2.6Hz, 1H), 7.63(d, J=8.6Hz, 2H), 7.34( d, J=8.4Hz, 2H), 7.29(d, J=8.5Hz, 2H), 7.15(d, J=8.2Hz, 2H), 6.71(d, J=7.5Hz, 1H), 2.43(s, 3H), 2.33(s, 3H). 13 C NMR (100M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com