Use of 3-hydroxy-4-pyridone high-molecular iron chelating agent
An iron chelating agent and polymer technology, which is applied in the field of 3-hydroxy-4-pyridone polymer iron chelating agents, can solve the problems of strong pathogenicity and multi-drug resistance, and achieves good compatibility and non-toxicity. Side effects, the effect of excellent antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
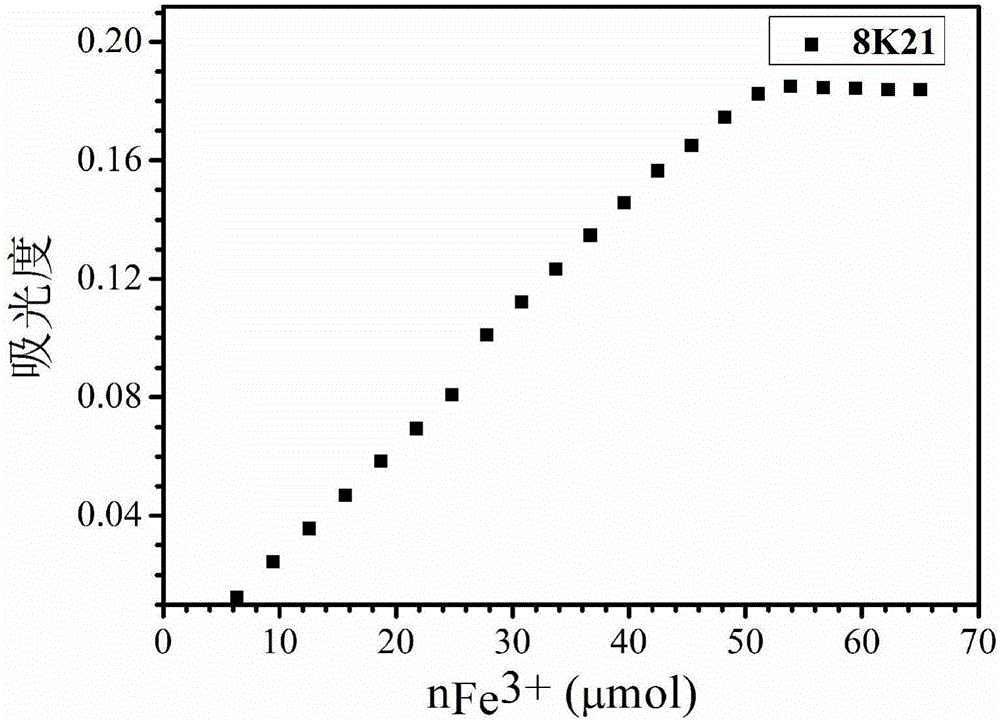
[0023] 0.2g polyglycidyl methacrylate PGMA1 (M n =12800g·mol -1 ) and 1.3g of 1-(amino-4,7,10-trioxytridecyl)-2-methyl-3-hydroxyl-4-(1H)-pyridone were added to a single-necked bottle, and then 8mL of anhydrous Dissolve N,N-dimethylformamide, then add 1 mL of triethylamine, keep at 55°C for 36 hours, add 80 mL of diethyl ether to the reaction liquid to precipitate the product, filter and dry the precipitate, put it into a dialysis bag for dialysis for 3 days (daily Change water four times, dialysis bag molecular weight cut-off is 3000) remove excess monomer and obtain macromolecular iron chelating agent 7K21, wherein the content of small molecular chelating agent in macromolecular iron chelating agent is about 70wt%. Dissolve 0.1 g of 7K21 in 1 mL of water, acetone, methyl ethyl ketone, N,N-dimethylformamide, and dimethyl sulfoxide to form a solution without any insoluble matter remaining in the solution. Calculate the number of repeating units in the polyglycidyl ether ester...
Embodiment 2
[0026] 0.2g polyglycidyl methacrylate PGMA2 (M n =10300g·mol -1) and 0.29g of 1-methylaminopropyl-2-methyl-3-hydroxyl-4-(1H)-pyridone were added to a one-necked bottle, then 8mL of anhydrous N,N-dimethylformamide was added to dissolve, and then Add 1 mL of triethylamine, keep it at 80°C for 2 hours, add 80 mL of diethyl ether to the reaction liquid to precipitate the product, filter the precipitate and dry it, put it into a dialysis bag for dialysis for 3 days (change distilled water four times a day, the molecular weight cut-off of the dialysis bag is 3000) Excessive monomers were removed to obtain the high-molecular iron chelating agent 8K41, wherein the content of the small-molecular chelating agent in the high-molecular iron chelating agent was about 58 wt%.
[0027] If other conditions remain unchanged, 0.2g of PGMA2 and 0.29g of 1-methylaminopropyl-2-methyl-3-hydroxyl-4-(1H)-pyridone in the reactant are replaced by 0.2g of polyglycidyl methacrylate Ether ester PGMA1, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com