Method for preparing block cationic water-borne polyurethane iron (III) chelates
A technology of block cation and polyurethane iron, which is applied in the field of preparation of block cationic waterborne polyurethane iron chelate, can solve the problems of low introduction rate, cumbersome steps, and many side reactions, and achieve adjustable structure and performance, post-treatment The effect of simplicity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
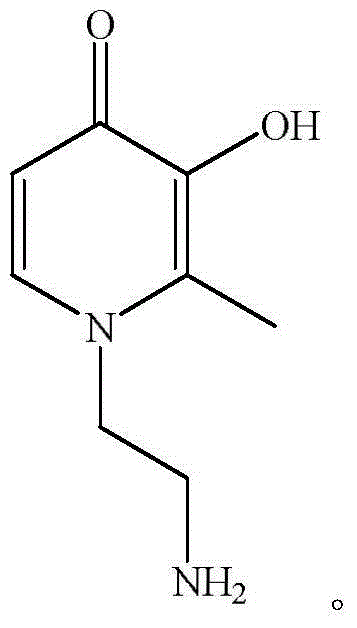
[0030] 33.9 grams of PPG (M n=2000) was dehydrated at 110°C for 0.5-1.5 hours, then added 26.4 grams of IPDI, reacted at 90°C for 2 hours, added 3.5 grams of DEG, reacted at 70°C for 2 hours, then added dropwise 7.6 grams of MDEA and 40mL at 50°C The mixed solution of butanone is dropped within 0.5-1 hour. After the drop, keep 65°C for 3 hours, add 0.045 grams of BHT and 1.2 grams of HEA and react at 60°C for 1 hour to obtain a double bond-terminated polyurethane prepolymer; Add 0.05 g of AIBN and 2.1 g of glycidyl methacrylate to the double bond-terminated polyurethane prepolymer, add 100 mL of butanone and react at 75 °C for 2 hours, add 2.8 g of 1-aminoethyl-2-methyl-3- Hydroxy-4-(1H)-pyridone reaction 75°C for 2 hours, then cool down to 30°C, add 3.98 g of acetic acid for 1-5 minutes, then add 181 mL of water under high-speed shear, stir for 5-30 minutes, and the reaction product At 45°C and 0.01MPa vacuum conditions, butanone was removed from the solvent to obtain a soli...
Embodiment 2
[0032] 50.0 g of PBA (M n =2000) was dehydrated at 110°C for 0.5-1.5 hours, then added 24.1 grams of TDI, reacted at 80°C for 2 hours, added 2.8 grams of BDO, reacted at 70°C for 2 hours, then added dropwise 9.0 grams of MDEA and 40 mL at 50°C The mixed solution of butanone is dropped within 0.5-1 hour. After the drop is completed, it is kept at 60°C for 3 hours, and 0.025 grams of HQ and 1.7 grams of HEMA are added to react at 60°C for 1 hour to obtain a double bond-terminated polyurethane prepolymer; Add 0.08 g of AIBN and 2.0 g of glycidyl methacrylate to the double bond-terminated polyurethane prepolymer, add 100 mL of methyl ethyl ketone and react at 65 °C for 2 hours, then add 2.37 g of 1-aminoethyl-2-methyl-3 -Hydroxy-4-(1H)-pyridone reaction at 75°C for 2 hours, then cool down to 30°C, add 4.9 g of acetic acid for 1-5 minutes, then add 215 mL of water under high-speed shear, stir for 5-30 minutes The product was desolventized butanone under 45°C and 0.01MPa vacuum con...
Embodiment 3
[0034] 50.0 grams of PPG (M n =2000) was dehydrated at 110°C for 0.5-1.5 hours, then added 38.87 grams of IPDI, reacted at 90°C for 2 hours, added 2.6 grams of EG, reacted at 70°C for 2 hours, then added dropwise 12.0 grams of MDEA and 40mL at 50°C The mixed solution of butanone is dropped within 0.5-1 hour. After the drop is completed, it is kept at 65°C for 3 hours, and 0.07 grams of BQ and 1.8 grams of HEMA are added to react at 60°C for 1 hour to obtain a double bond-terminated polyurethane prepolymer; Add 0.056 g of AIBN and 5.6 g of glycidyl methacrylate to the double bond-terminated polyurethane prepolymer, add 100 mL of butanone and react at 65 °C for 2 hours, then add 6.6 g of 1-aminoethyl-2-methyl-3 -Hydroxy-4-(1H)-pyridone reacted at 75°C for 2 hours, then cooled to 30°C, added 7.2 g of acetic acid for 1-5 minutes, then added 274 mL of water under high-speed shear, and stirred for 5-30 minutes. The product was desolventized butanone under 45°C and 0.01MPa vacuum co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com