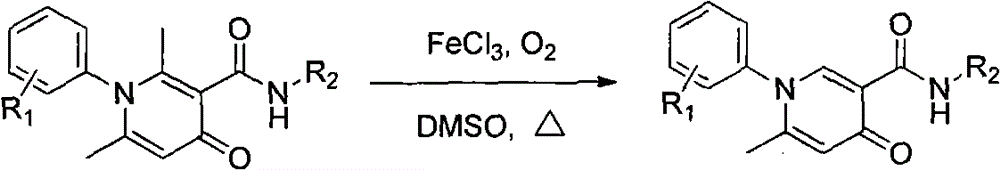Simple and effective method for high-selectivity removal of alpha-monomethyl of 2,6-dimethyl-4-pyridone derivative
A high-selectivity, pyridone technology, applied in the field of removal 2, can solve the problems of less than 5% yield, excessive use of strong base and strong acid at high reaction temperature, etc., and achieves good catalytic effect, few experimental steps, and low volatility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] In a 25 mL round bottom flask was added 2,6-dimethyl-4-oxo-N,1-di-p-tolyl-1,4-dihydropyridine-3-carboxamide (346 mg, 1.0 mmol), anhydrous FeCl 3 (195mg, 1.2mmol) and 4.0mL DMSO, heat the reaction in an oxygen (1.0atm) atmosphere, the reaction process is monitored by LC-MS, after the reaction, the system is naturally cooled to room temperature, and 20mL of water is added to the reaction system and stirred at room temperature. An insoluble solid precipitated out and was filtered by suction. The insoluble solid was purified by column chromatography to obtain 6-methyl-4-oxo-N,1-di-p-tolyl-1,4-dihydropyridine-3-carboxamide (269 mg, 81%).
[0020] 6-Methyl-4-oxo-N, 1-di-p-tolyl-1, 4-dihydropyridine-3-carboxamide
[0021] Colorless crystal.mp: 242-244℃; Yield: 81%, 1 H NMR (400MHz, CDCl 3 )δ12.42(s, 1H), 8.54(s, 1H), 7.63(d, J=7.5Hz, 2H), 7.33(d, J=7.5Hz, 2H), 7.16(d, J=8.7Hz, 2H), 7.14(d, J=8.0Hz, 2H), 6.54(s, 1H), 2.45(s, 3H), 2.32(s, 3H), 2.08(s, 3H). 13 C NMR (100MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com