Method for preparing 2-fluoro-4-bromo trifluoromethoxyphenyl
A technology of bromotrifluoromethoxybenzene and trifluoromethoxybenzene is applied in the field of preparation of 2-fluoro-4-bromotrifluoromethoxybenzene, and can solve the problems of poor selectivity of metal reagents or Grignard reagents, fluorine The chemical agent process and equipment are difficult to realize, and it is difficult to industrialize production, so as to achieve the effect of less by-products, low cost, and easy industrialization in the synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
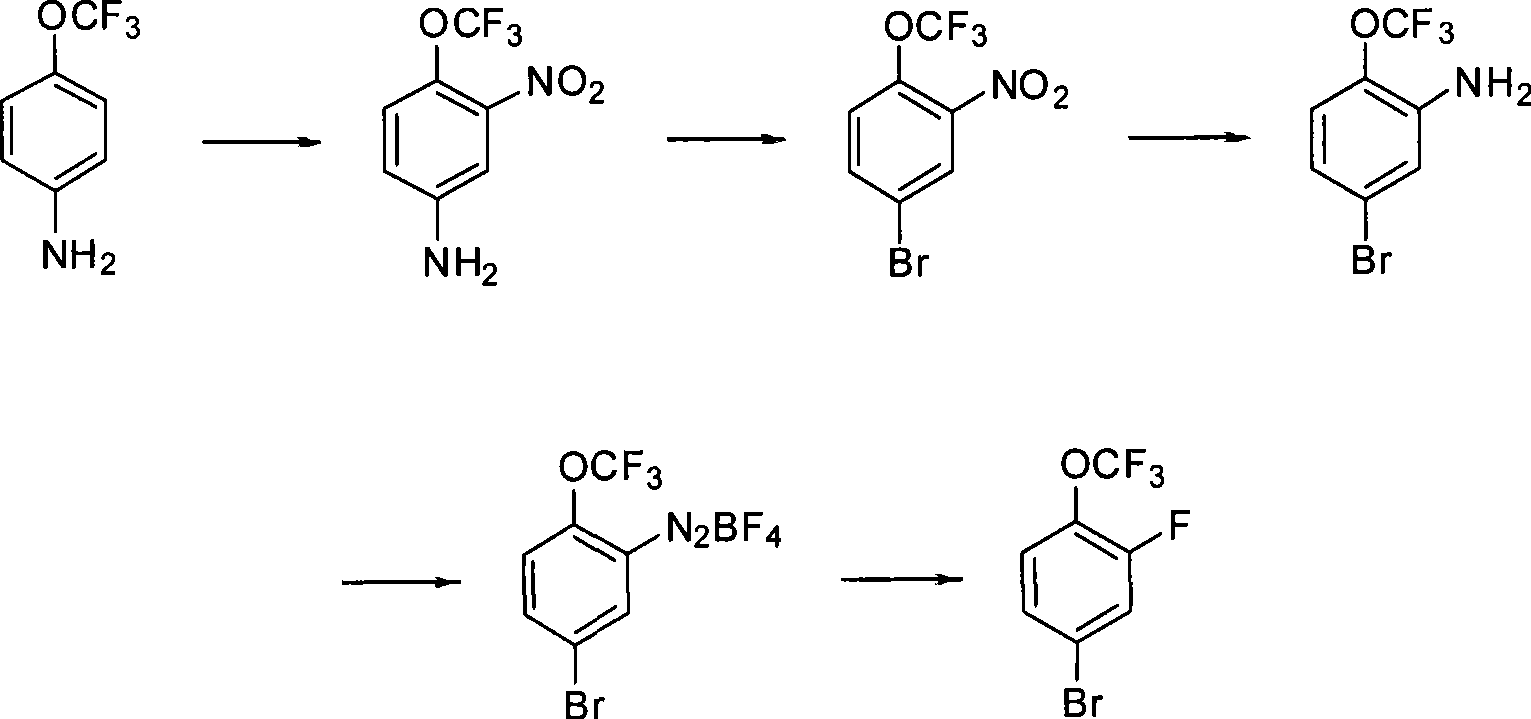
[0012] Specifically, the preparation method of 2-fluoro-4-bromotrifluoromethoxybenzene comprises the following steps:
[0013] (1) Nitrate and nitrate 4-aminotrifluoromethoxybenzene to obtain 2-nitro-4-amino-trifluoromethoxybenzene;
[0014] (2) The obtained 2-nitro-4-amino-trifluoromethoxybenzene is subjected to diazotization and Sandmeyer reaction to obtain 2-nitro-4-bromotrifluoromethoxybenzene;
[0015] (3) The obtained 2-nitro-4-bromotrifluoromethoxybenzene is subjected to reduction, diazotization and Schiemann reaction to obtain 2-fluoro-4-bromotrifluoromethoxybenzene.
[0016] The synthesis route of the above-mentioned preparation method can be represented by the following reaction formula, for example.
[0017]
[0018] In the above method, preferably, the nitration reaction in the step (1) is carried out at a temperature range of -30°C to 50°C, more preferably, the nitration reaction in the step (1) is carried out at a temperature of -10°C to within a temperature...
Embodiment 1
[0033] Preparation of 2-nitro-4-aminotrifluoromethoxybenzene
[0034] At an internal temperature of -10°C to 10°C, add concentrated H 2 SO 4 (96%; 360g; 3.5mol) 25ml (0.555mol) of 95% nitric acid, 4-aminotrifluoromethoxybenzene (99.4%; 88.5g; 0.5mol), the reaction is exothermic, and the system is light yellow after dropping Thick, keep the internal temperature at -10°C ~ -5°C and stir until the raw materials are basically reacted. Pour into 500g+100ml of ice water, stir and filter with suction to obtain 9.5g of dark brown solid with a content of 89%. The filter cake is washed with 100ml×2 water and dried to obtain 140g of light yellow wet solid, which is dried to obtain 94.5g , organic phase chromatographic analysis, content 97.7%. The boiling point is 200°C.
Embodiment 2
[0036] Preparation of 2-nitro-4-bromotrifluoromethoxybenzene
[0037] Raise the internal temperature to 50°C, add CuBr 15g, HBr (48%) 420g (2.4mol), water 85ml, 2-nitro-4-aminotrifluoromethoxybenzene (98%) 108.5 g (0.48mol), NaNO 2 solution (NaNO 2 50g; 0.72mol / 120ml water), the reaction is exothermic, and bubbles will come out immediately, the reaction is basically kept at 50-55°C, and the sample is taken after the drop is completed, and there is no raw material. The system is layered, and the organic phase is washed with 20ml of 25% concentrated ammonia water to make 100ml of dilute ammonia water, which is neutral, solvent, distilled (10mmHg, top temperature 115°C ~ 117°C), and 120g of product is distilled out, analyzed by organic phase chromatography, the content 88.9%. The yield was 77.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com