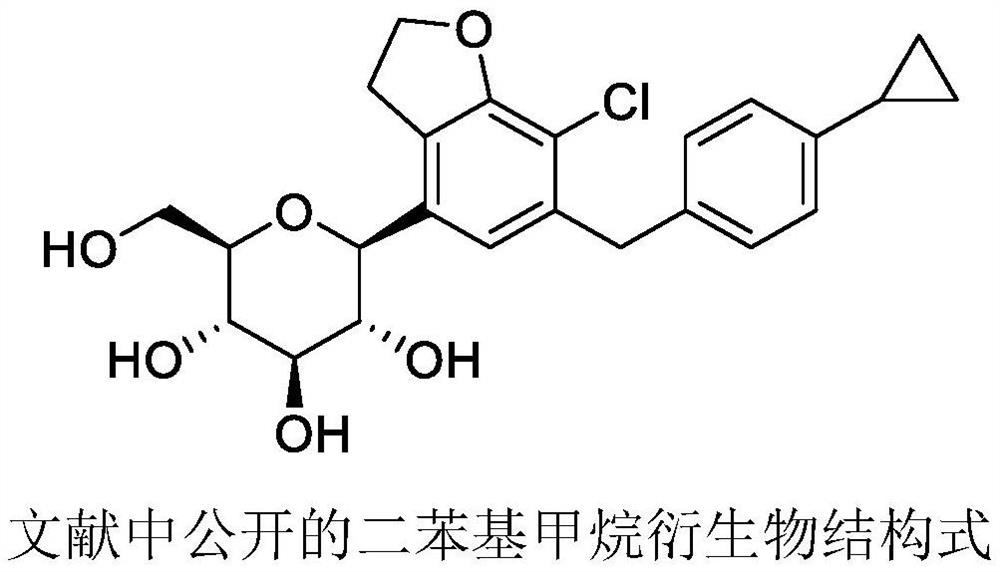
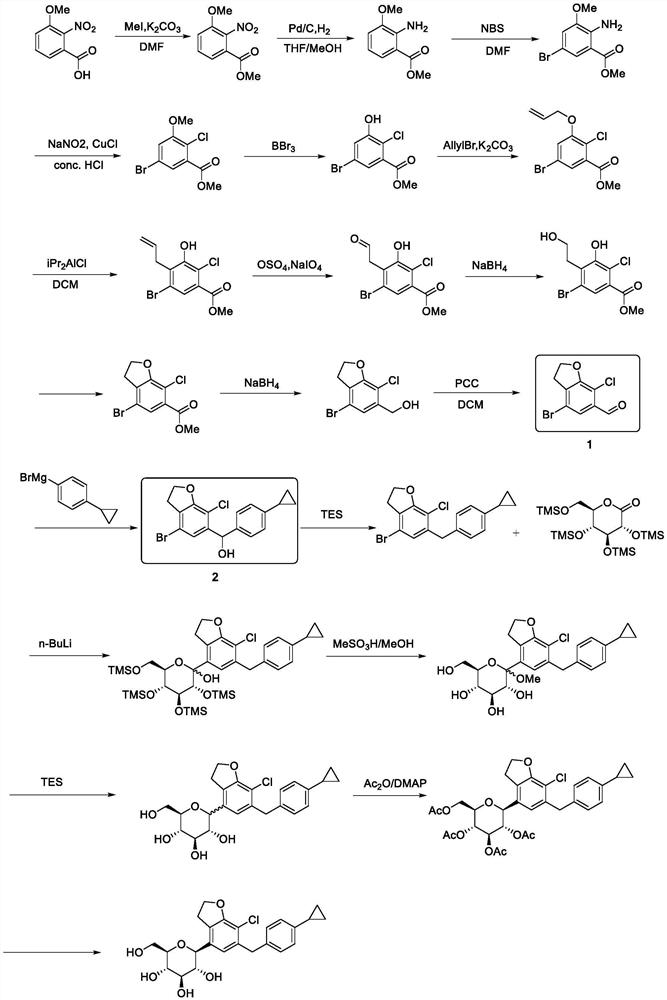
Synthetic processes of intermediates for preparation of SGLT inhibitor
A technology for reagents and compounds, applied in the field of synthesizing SGLT inhibitors, can solve the problems of difficulty in meeting the needs of the pharmaceutical industry, high production costs, complex overall operations, etc., and achieve the effects of low cost, short route and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of 4,6-dibromo-2,3-dihydrobenzofuran-7-aniline
[0032]
[0033] In a 1000mL reaction flask, add 8.7g 2,3-dihydrobenzofuran-7-aniline, 200mL ethyl acetate, and then add 41.4g bromine (here bromine The added amount of bromine is the result of experimental optimization, if the added amount of bromine is too little, the reaction will not be complete; if the added amount is too high, the system will become complicated and the by-products will increase; controlling the reaction temperature is mainly to prevent the by-products from increasing). After the addition is complete, filter. Filter cake is added in the mixed solvent of the ethyl acetate of 100mL water and 100mL, is added dropwise 10% sodium hydroxide solution, adjusts pH between 8-10 (system is acidic after reaction, part product becomes salt, adjusts pH= 8-10 is for dissociation. If the pH is too low, the dissociation is not complete; if it is too high, more alkali will be consumed). The or...
Embodiment 2
[0034] Example 2: Synthesis of 4,6-dibromo-2,3-dihydrobenzofuran-7-aniline
[0035] In a 100mL reaction flask, add 5g of 2,3-dihydrobenzofuran-7-aniline, 100mL of N,N-dimethylformamide, cool to -5-0°C, and add 13.17g of N-bromosuccinimide (the advantage of N-bromosuccinimide is: N-bromosuccinimide is solid, easy to calculate and control; bromine is corrosive liquid, irritating , weighing and use are inconvenient), stirred and reacted for 30 minutes. The reaction solution was poured into 200 mL of water, extracted twice with ethyl acetate, the combined organic phase was washed with 10% sodium hydroxide, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 8.47 g of 4,6-dibromo-2,3 -Dihydrobenzofuran-7-aniline, yield 78.2%.
Embodiment 3
[0036] Example 3: Synthesis of 4,6-dibromo-7-chloro-2,3-dihydrobenzofuran
[0037]
[0038] Add 10g of 4,6-dibromo-2,3-dihydrobenzofuran-7-aniline and 50mL of concentrated hydrochloric acid into a 250mL reaction flask, cool down to 0-5°C, add sodium nitrite solution (2.47g dissolved in 8mL of water). After the dropwise addition was completed, the reaction was incubated for 30 minutes, then 6.76 g of cuprous chloride was added in batches, and the temperature was raised to room temperature for 2 hours. Ethyl acetate was added for extraction, the organic layer was washed with 5% sodium hydroxide solution, concentrated to obtain a crude product, and 9.01 g of 4,6-dibromo-7-chloro-2,3-dihydrobenzofuran was obtained by silica gel column chromatography , Yield 84.5%, white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com