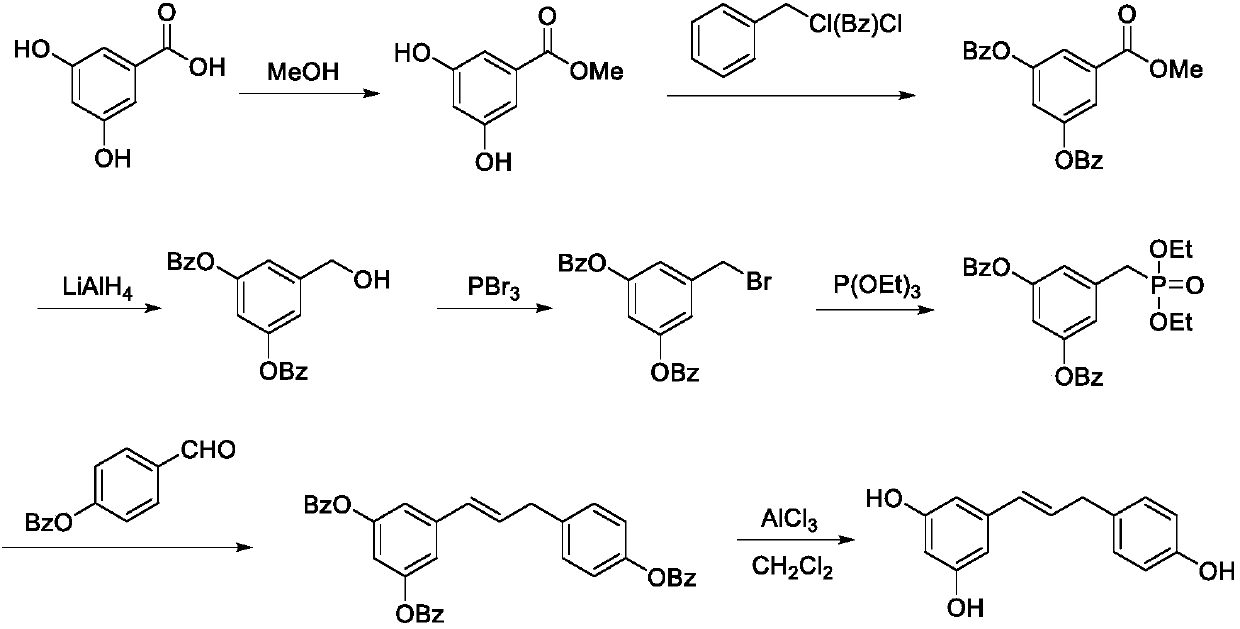
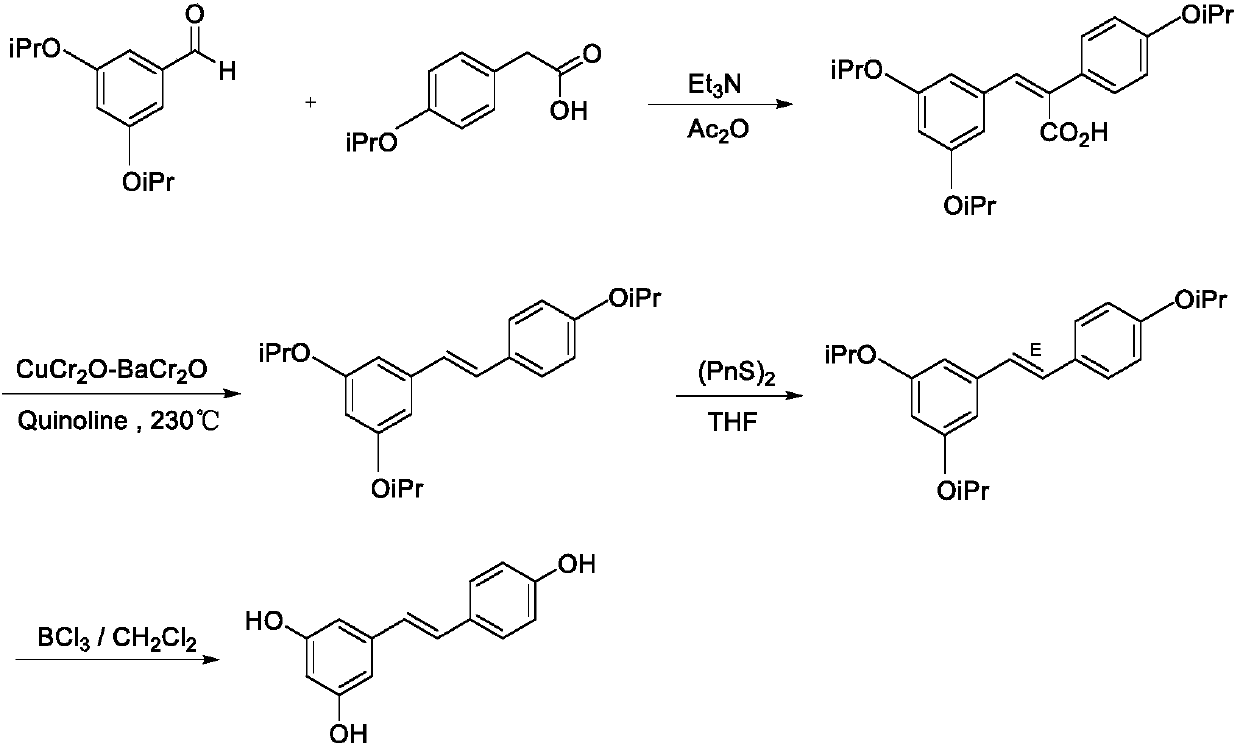
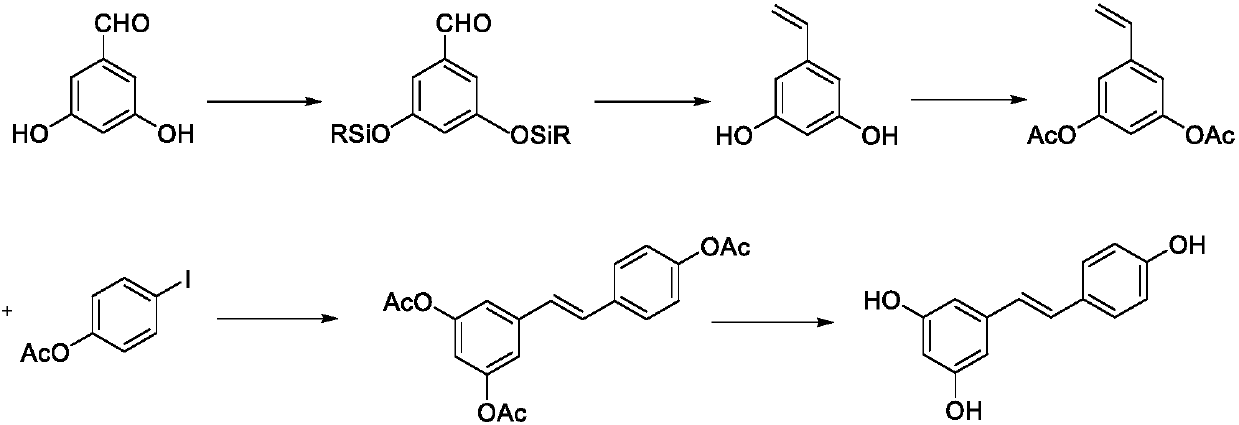
Synthesis method of resveratrol
A synthetic method, the technology of resveratrol, which is applied in the field of natural product synthesis, can solve the problems of high price and achieve the effect of simple post-treatment, simple operation and post-treatment, and high trans-stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] Experimental part
[0033] (1) Synthesis of 12
[0034] Add 3,5-dimethoxybenzoic acid (10.0 g (gram), 55 mmol (mmol)) into a 500 mL (milliliter) round bottom flask, then add 50 mL of thionyl chloride to dissolve it, and heat at 90° C. ) Reflux for 2h (hours) (TLC (thin layer chromatography) tracking). After the reaction, the thionyl chloride was evaporated to dryness under reduced pressure at 60°C to obtain 11 g of brown oily substance 12, which was directly used in the next step without purification.
[0035] (2) Synthesis of 13
[0036] Add 50mL of ammonia water to a 500mL round-bottomed flask, ice-bath for 20 minutes, dissolve 12 (11.0g, 55mmol) in 50mL of acetonitrile and slowly add it dropwise to the three-necked flask, during which a large amount of white solids are produced. After the addition is complete, react at room temperature for 1.5h ( TLC trace). After the reaction was completed, it was transferred to a round-bottomed flask, and the acetonitrile was e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com