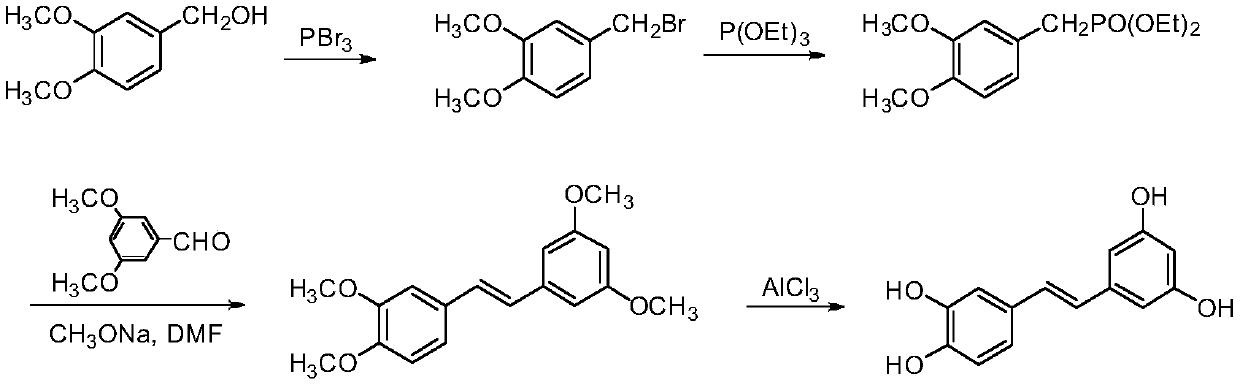
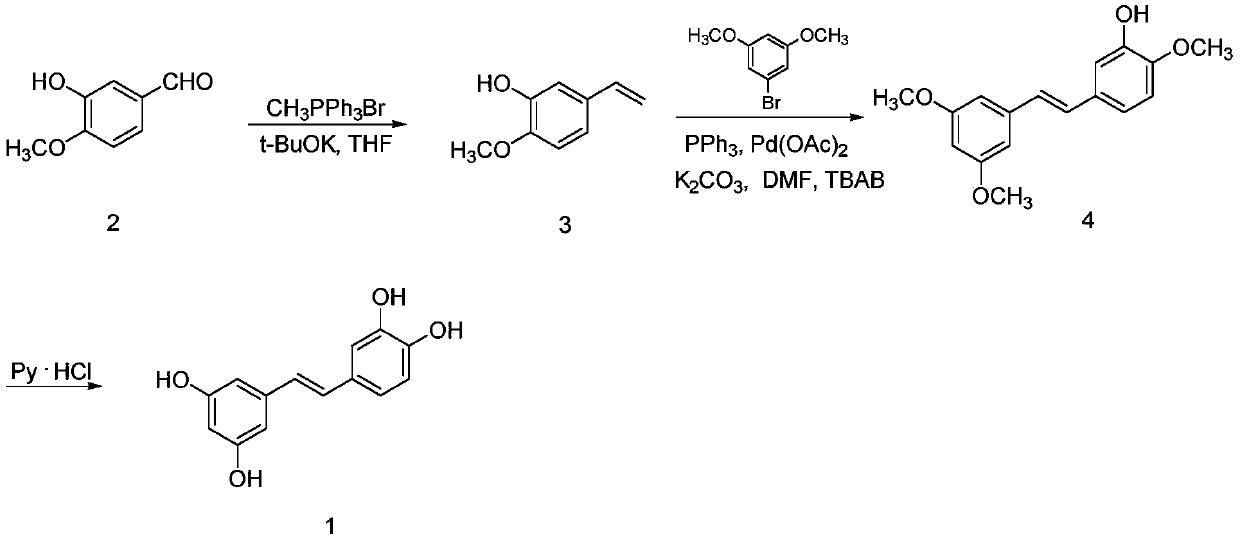
A kind of synthetic method of picatanol
A synthetic method, the technology of picatanol, applied in the direction of organic chemical methods, chemical instruments and methods, skin care preparations, etc., can solve the problem that biological extraction is difficult to meet market demand, the structure of the target product is not single, and the chemical synthesis operation is complex, etc. problem, to achieve the effect of high trans stereoselectivity, good development prospects and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of 3-hydroxy-4-methoxystyrene
[0027] Add 3-hydroxyl-4-methoxybenzaldehyde (2.0g, 13.14mmol) in 100mL round bottom flask, then add 50mL THF to dissolve it, then add potassium tert-butoxide (4.4g, 39.21mmol), finally Add methyltriphenylphosphine bromide (12.7 g, 35.55 mmol) and heat at 70° C. (Celsius). After 7 hours, TLC detects that the reaction is complete. Cool to room temperature, add 50mL of water, extract with ethyl acetate (50mL×3), combine the organic phases, wash with saturated brine (100mL×3), and finally dry with anhydrous sodium sulfate, spin dry to obtain 2.6g of yellow oil, Purified by column chromatography (ethyl acetate:petroleum ether=1:30, 1 L) to obtain 1.2 g of 3-hydroxy-4-methoxystyrene as a white solid, with a yield of 60.91%. 1 H NMR (300MHz, CDCl 3 )δ: 3.77 (s, 3H), 5.08 (d, J = 12.0Hz, 1H), 5.66 (d, J = 18.0Hz, 1H), 6.68 (dd, J = 18.0and12.0Hz, 1H), 6.88 ( m,3H), 9.00(s,1H).
[0028] (2) Synthesis of E-3-hydroxy-3',4,5'-trimet...
Embodiment 2
[0033]In step (1), the molar ratio of 3-hydroxy-4-methoxybenzaldehyde, potassium tert-butoxide and methyltriphenylphosphine bromide is: 1:2.5:2.5, reaction temperature: 75°C, reaction time: 6h.
[0034] In step (2), 3-hydroxyl-4-methoxystyrene, 3,5-dimethoxybromobenzene, salt of wormwood, tetrabutylammonium bromide, the mol ratio of palladium acetate and triphenylphosphine are: 1:1.2:2.0:0.08:0.06:0.08, the reaction temperature is: 110°C, and the reaction time is 6.5h.
[0035] In step (3), the molar ratio of pyridine hydrochloride to E-3-hydroxyl-3',4,5'-trimethoxystilbene is 60:1, the reaction temperature is 200°C, and the reaction time is: 1.5h.
Embodiment 3
[0037] In step (1), the molar ratio of 3-hydroxyl-4-methoxybenzaldehyde, potassium tert-butoxide and methyltriphenylphosphine bromide is: 1:3.5:3.5, reaction temperature: 80°C, reaction time: 8h.
[0038] In step (2), 3-hydroxyl-4-methoxystyrene, 3,5-dimethoxybromobenzene, salt of wormwood, tetrabutylammonium bromide, the mol ratio of palladium acetate and triphenylphosphine are: 1:1.5:2.2:0.12:0.08:0.1, the reaction temperature is: 130°C, and the reaction time is 9h.
[0039] In step (3), the molar ratio of pyridine hydrochloride to E-3-hydroxy-3',4,5'-trimethoxystilbene is 80:1, the reaction temperature is 260°C, and the reaction time is: 0.5h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com