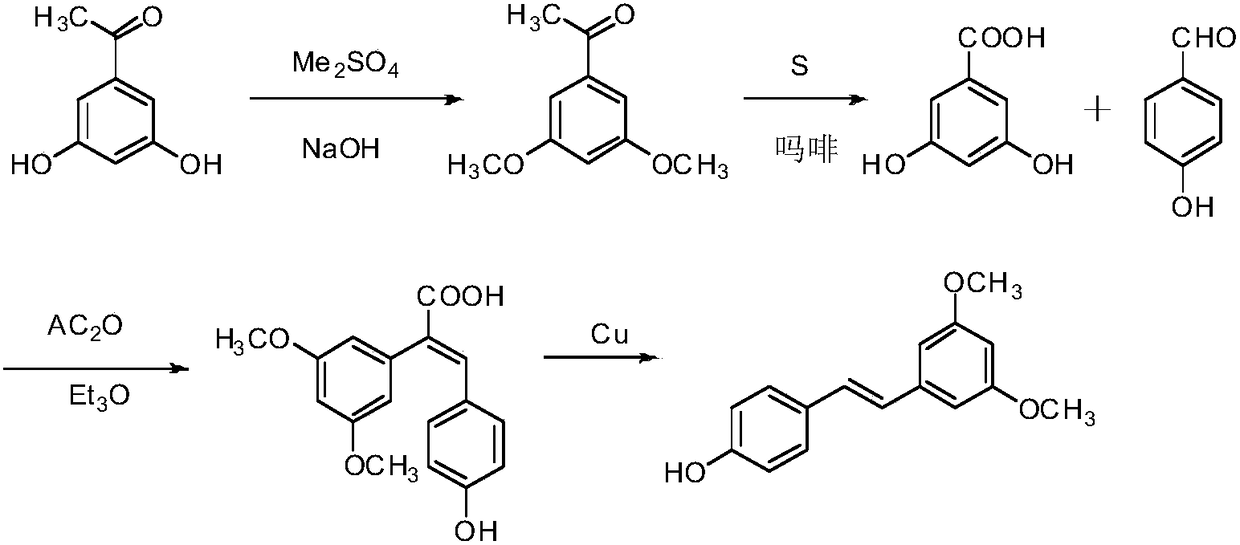
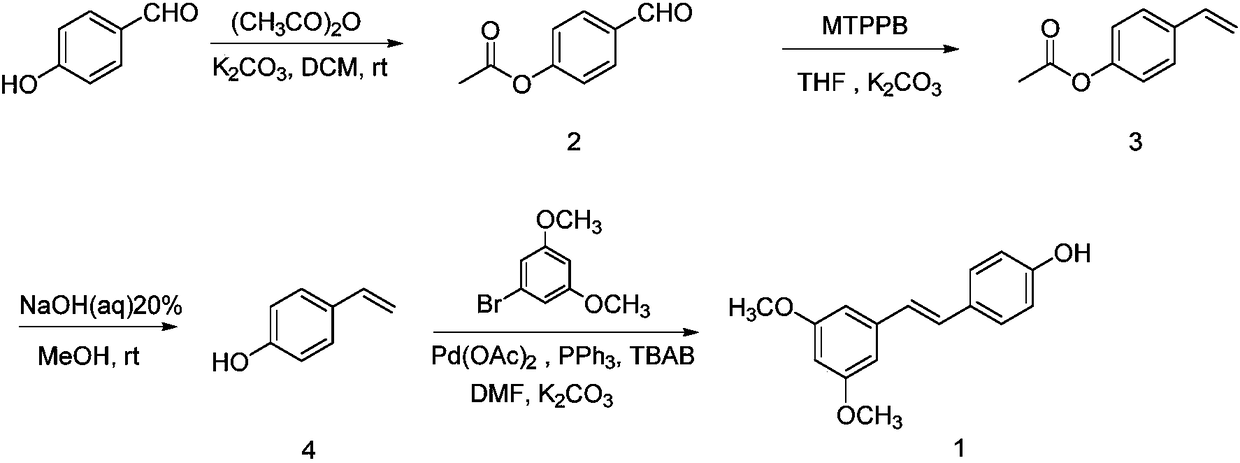
Synthesis method of pterostilbene
A synthesis method and technology of pterostilbene are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of non-single structure of target products, complex synthesis steps and high cost, and achieve good development prospects and simple operation. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of 2
[0028] Add p-hydroxybenzaldehyde (2.0g (gram), 16.38mmol (mmol)) to a 50mL (milliliter) round bottom flask, then add 20mL of dichloromethane to dissolve it, and then add potassium carbonate (2.5g, 18.00mmol ), and finally acetic anhydride (1.8mL, 18.00mmol) was slowly added dropwise, and after 2h (hour) reaction at room temperature, it was detected by TLC (thin layer chromatography), and the reaction was completed. Potassium carbonate was removed by suction filtration, and dichloromethane was spun off from the filtrate under reduced pressure at 50°C to obtain 2.6 g of light yellow oily substance 4-acetoxybenzaldehyde (2), with a yield of 96.73%. 1 H NMR (400MHz, CDCl 3 ) δ: 2.34 (s, 3H), 7.28 (d, J=8.0Hz, 2H), 7.92 (d, J=12.0Hz, 2H), 9.99 (s, 1H).
[0029] (2) Synthesis of 3
[0030] Add 4-acetoxybenzaldehyde (2.0g, 12.18mmol) in a 100mL round bottom flask, then add 50mL tetrahydrofuran to dissolve it, then add potassium carbonate (1.9g, 13.75mmo...
Embodiment 2
[0036] In the step (1), the molar ratio of hydroxybenzaldehyde, potassium carbonate and acetic anhydride is 1:1.3:1.3, the reaction time is 3 hours, and the yield is 95.82%.
[0037] In step (2), the molar ratio of 4-acetoxybenzaldehyde, potassium carbonate and methyl triphenylphosphine bromide is: 1:1.3:1.3, the reaction temperature is 80°C, the reaction time is 30h, and the yield is 58.66%
[0038] The mass volume ratio of 4-acetoxystyrene to sodium hydroxide in step (3) is: 1:12, the reaction time is 1h, and the yield is 94.55%
[0039] In step (4), the molar ratio of 4-hydroxystyrene, 3,5-dimethoxybromobenzene, potassium carbonate, tetrabutylammonium bromide, palladium acetate and triphenylphosphine is 1:1.2:2.4:0.05 :0.04:0.1, reaction time 10h, reaction temperature 110°C, yield 55.33%
Embodiment 3
[0041] In step (1), the molar ratio of hydroxybenzaldehyde, potassium carbonate and acetic anhydride is 1:1:1, the reaction time is 2.5 hours, and the yield is 94.68%.
[0042] In step (2), the molar ratio of 4-acetoxybenzaldehyde, potassium carbonate and methyltriphenylphosphine bromide is 1:1:1, the reaction temperature is 66° C., the reaction time is 25 hours, and the yield is 53.78%.
[0043] In step (3), the mass volume ratio of 4-acetoxystyrene to sodium hydroxide is 1:8, the reaction time is 1.5 h, and the yield is 93.88%.
[0044] In step (4), the molar ratio of 4-hydroxystyrene, 3,5-dimethoxybromobenzene, potassium carbonate, tetrabutylammonium bromide, palladium acetate and triphenylphosphine is 1:1.5:2.1:0.12 :0.06:0.15. The reaction time was 12 hours, the reaction temperature was 130°C, and the yield was 53.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com