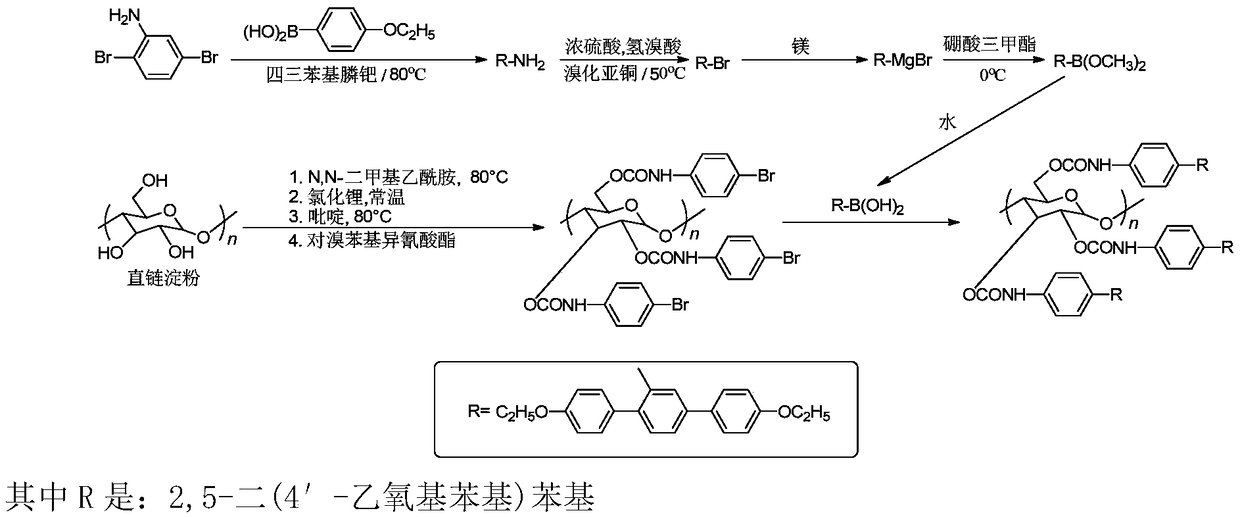
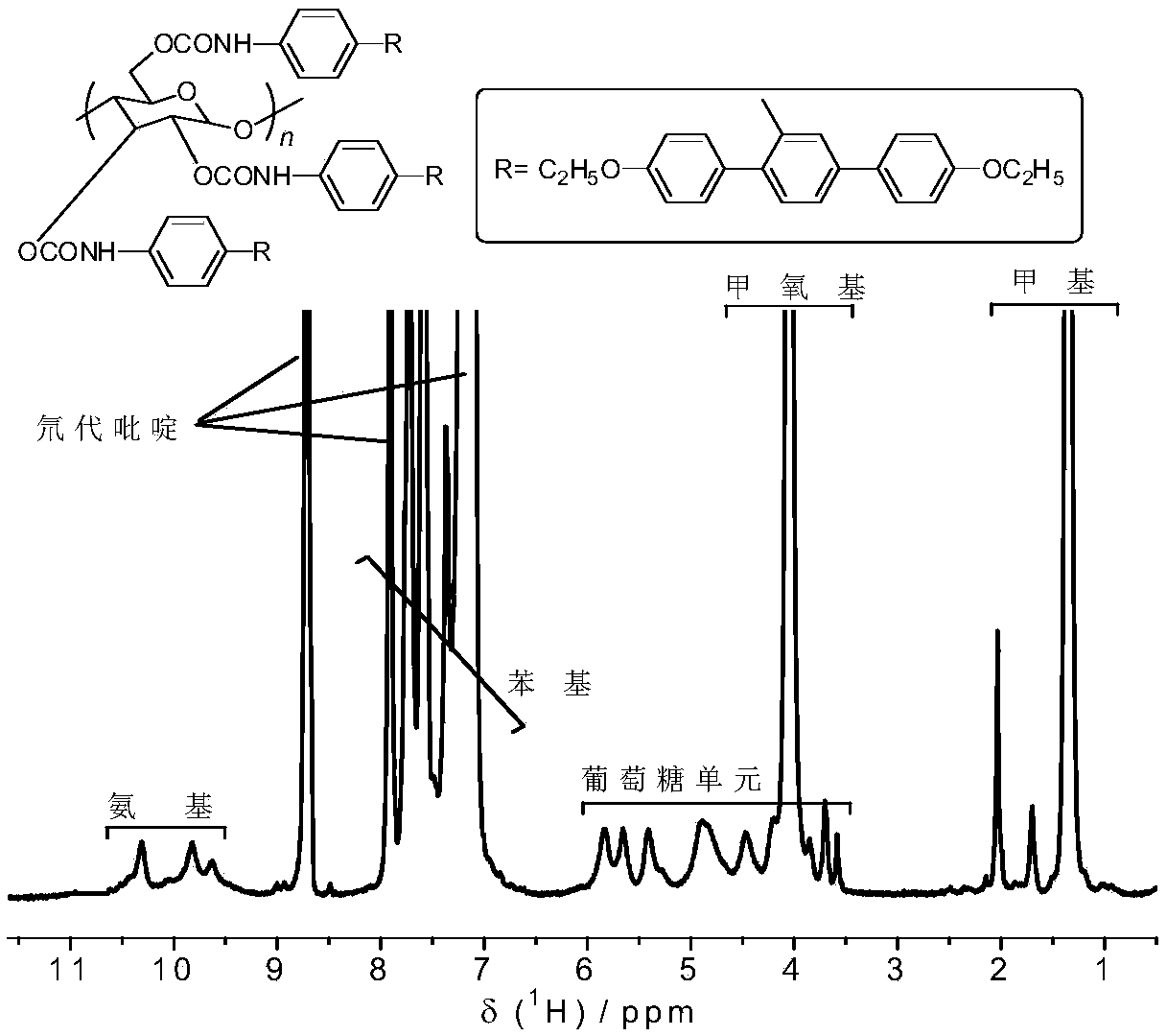
Preparation method of chiral fluorescence sensor with large-volume side group amylase derivative
A fluorescence sensor and amylose technology are applied in the field of preparation of functional polymer materials to achieve the effects of high yield, simple synthesis process and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The following examples describe the present invention in more detail.
[0020] 1. Add 24mmol 4-ethoxyphenylboronic acid, 10mmol 2,5-dibromoaniline, 60mmol sodium carbonate, 0.4mmol tetrakis (triphenylphosphine) palladium into a two-neck flask, and then add 75ml volume ratio 2 / 1 A mixed solution of dioxane and water; the reaction system is heated to 80° C. and stirred for 32 hours, and then the reaction ends. Then the temperature of the reaction system was lowered to room temperature, and the crude product was obtained after removing the solvent. The crude product was purified by column chromatography to obtain pure light yellow powder of 2,5-bis(4'-ethoxyphenyl)aniline. The yield was 83.3%.
[0021] 2. Take 9mmol of 2,5-bis(4'-ethoxyphenyl)aniline in a two-necked flask, add 14.6mL of a 20wt% sulfuric acid and water mixed solution, heat at 60℃ for 2h until the sample is completely dissolved; Slowly add 3ml of 3.3mol / L sodium nitrite solution to the reaction flask under ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com