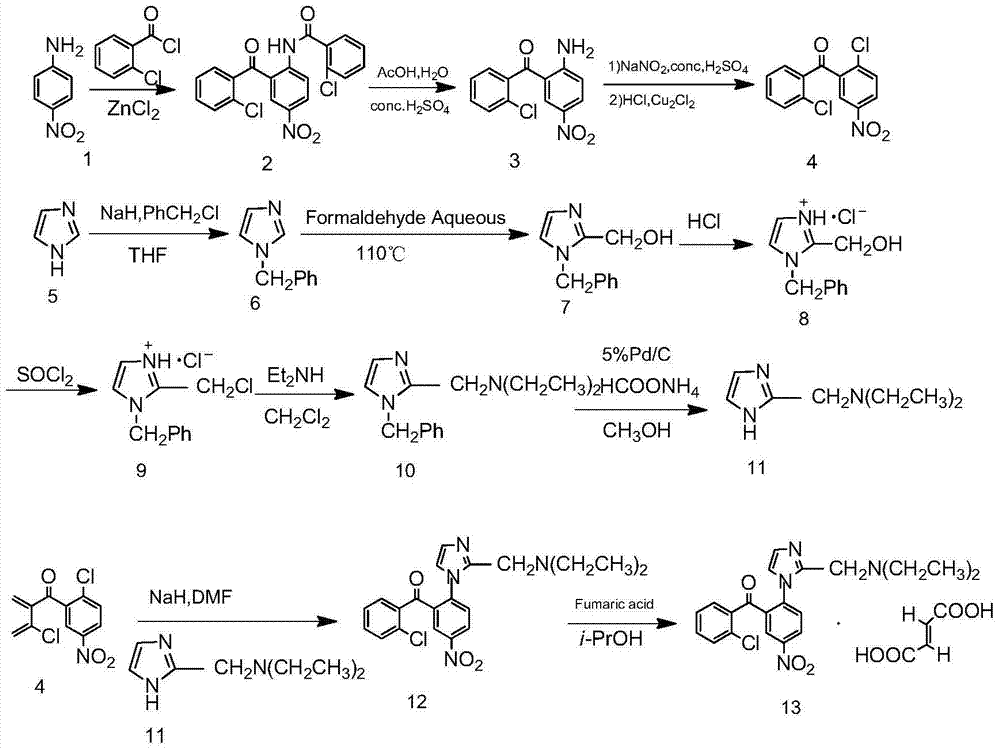
Synthesis process of nizofenone fumarate
A technology of nizophenone fumarate and process, which is applied in the field of medicine, and can solve problems such as polluting the environment, poor selectivity, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Illustrate with respect to summary of the invention below, summary of the invention includes cited example but not limited to following example:
[0021] (1) Preparation of 2′-chloro-2-amino-5-nitrobenzophenone (1)
[0022] Reflux 34g (0.25mol) of zinc chloride and 100mL of thionyl chloride for 15 minutes, then evaporate the thionyl chloride to dryness to obtain a white powder, add 60.8mL (0.50mol) of o-chlorobenzoyl chloride, and heat up to 140°C, add 27.6g (0.20mol) p-nitroaniline in batches, after 0.5h, heat up to 205°C, react for 1h, add dropwise glacial acetic acid aqueous solution (78mL glacial acetic acid, 54mL water), 102mL concentrated sulfuric acid, reflux 4h, cooled, and left overnight. Transfer the solid to a 2000mL three-neck flask, add 300mL each of toluene, ethyl acetate, and water and heat to 70°C, pour the black liquid into a 2000mL separatory funnel, remove the water layer, and use (160mL*3) 4mol of the organic phase in turn / L hydrochloric acid, (16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com