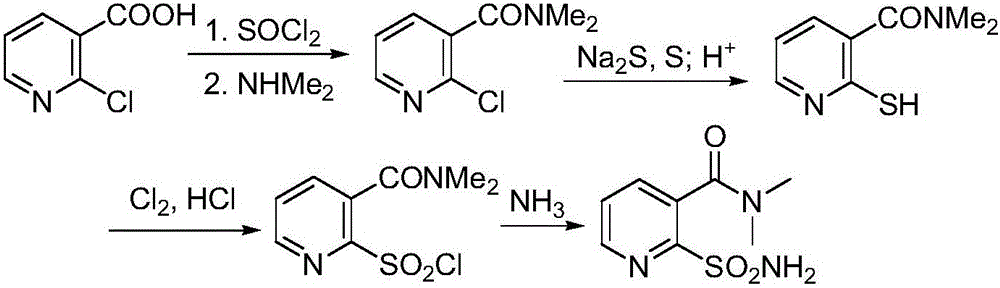
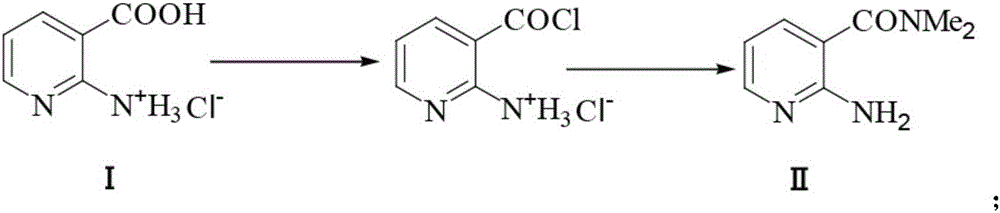
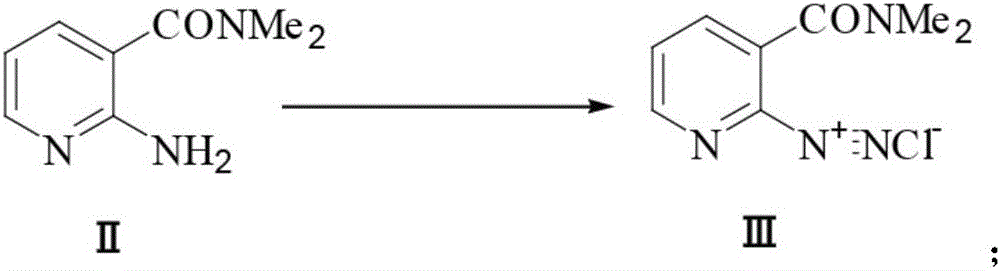
Preparation method of 2-aminosulfonyl-N,N-dimethylnicotinamide
A technology of dimethylnicotinamide and aminosulfonyl, which is applied in the field of preparation of 2-aminosulfonyl-N,N-dimethylnicotinamide, can solve problems such as poor process stability, low cost, blackened sulfur, etc. problems, to avoid the use of polysulfides, to achieve good yield and quality, and to achieve mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1) Synthesis of intermediate II (2-amino-N,N-dimethylnicotinamide):
[0062] 17.46g (100mmol) of 2-aminonicotinic acid hydrochloride and 100mL of chlorobenzene were mixed in a 250mL round bottom flask, stirred at room temperature, and 14.86g (125mmol) of thionyl chloride was added dropwise. After the dropwise addition, the system was heated to Stir at 50-55°C for 1 h, and distill under reduced pressure to remove excess thionyl chloride and half chlorobenzene, and maintain the system volume at about 60 mL. Heat and stir to raise the temperature of the system to 80°C, slowly pass in about 5.4g (120mmol) of dimethylamine gas, and keep stirring at this temperature for 30 minutes after passing through, then cool down to 0°C, a large amount of solids are precipitated, and suction filtered to obtain 2 -Amino-N,N-dimethylnicotinamide 15.44g, yield 93.6%, content 96.7%, m.p.83-85°C.
[0063] 2) Synthesis of diazonium salt III (2-diazonium chloride-N,N-dimethylnicotinamide):
...
Embodiment 2
[0070] 1) Synthesis of intermediate (2-amino-N,N-dimethylnicotinamide):
[0071] 17.46g (100mmol) of 2-aminonicotinic acid hydrochloride and 100mL of chlorobenzene were mixed in a 250mL round bottom flask, stirred at room temperature, and 14.86g (125mmol) of thionyl chloride was added dropwise. After the dropwise addition, the system was heated to Stir at 50-55°C for 1 h, and distill under reduced pressure to remove excess thionyl chloride and half chlorobenzene, and maintain the system volume at about 60 mL. Heat and stir to raise the temperature of the system to 80°C, slowly pass in about 5.4g (120mmol) of dimethylamine gas, and keep stirring at this temperature for 30 minutes after passing through, then cool down to 0°C, a large amount of solids are precipitated, and suction filtered to obtain 2 -Amino-N,N-dimethylnicotinamide 15.44g, yield 93.6%, content 96.7%, m.p.83-85°C.
[0072] 2) Synthesis of diazonium salt III (2-diazonium chloride-N,N-dimethylnicotinamide):
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com