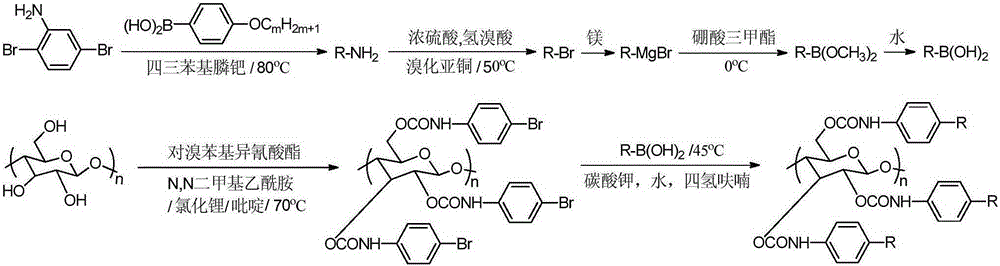
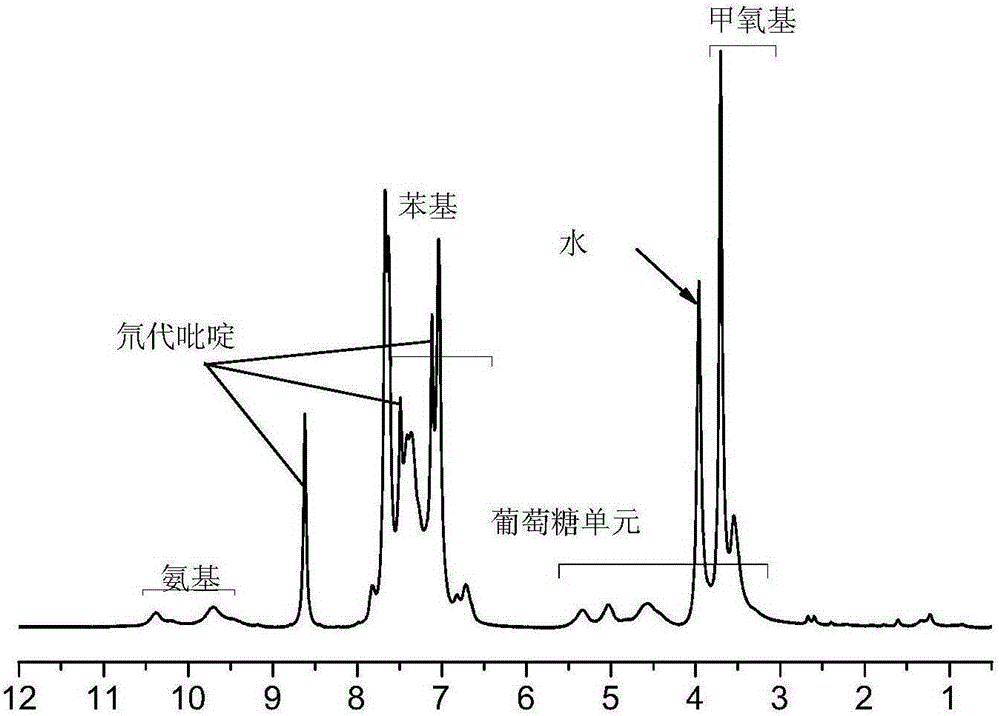
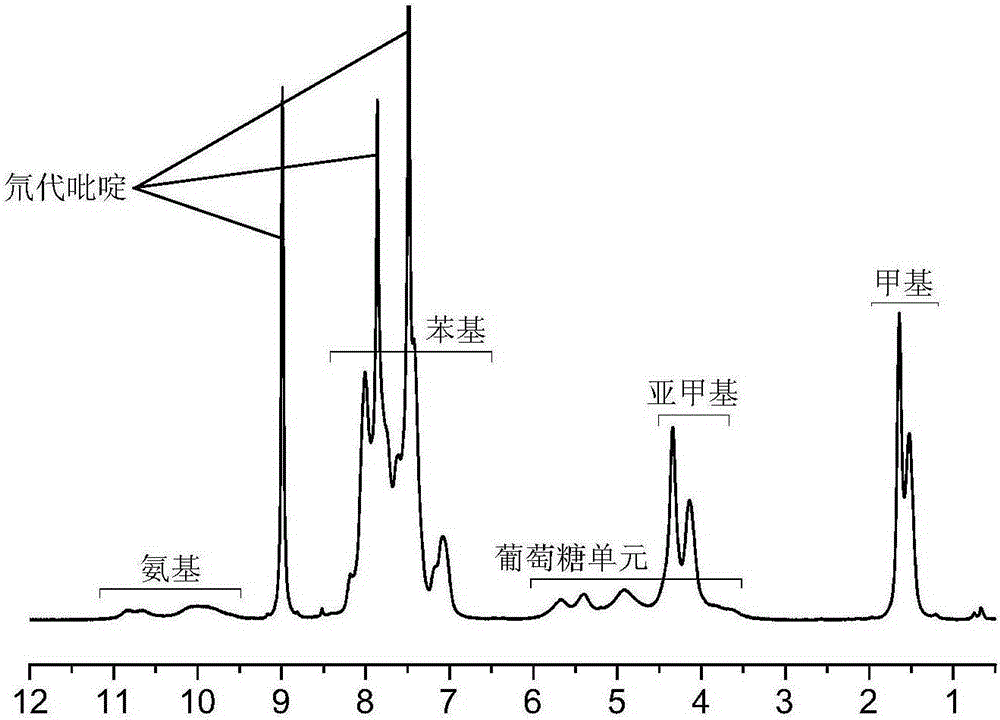
Synthetic method for cellulose derivative with large volume liquid crystal unit side group
A technology of liquid crystal unit and cellulose, which is applied in the field of synthesis of cellulose derivatives with side groups of large-volume liquid crystal units, can solve the problems of small substituents, and achieve the effects of easy control, mature synthesis process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0029] 1. Take 15mmol 2,5-dibromoaniline, 36mmol p-methoxyphenylboronic acid, 0.09mmol sodium carbonate and 0.6mmol tetrakistriphenylphosphine palladium and vacuum dry for 0.5h, then mix in 75ml dioxane and 37.5ml water Stir and reflux at 80°C for 30 hours to stop the reaction; cool to room temperature, remove the solvent by rotary evaporation, add n-hexane to filter and wash; purify the crude product by column chromatography to obtain pure 2,5-bis(4'-methoxy phenyl) aniline with a yield of 80%.
[0030] 2. Take 1.6mmol of the above-mentioned intermediate product and put it into reaction bottle 1, add 14.0ml of water and 0.26ml of 98% concentrated sulfuric acid mixture, stir and reflux at 60°C for 1.5h; then add 1ml of 1.76mol / L sodium nitrite at 0°C Solution, stirred and refluxed for 1h; vacuum-dried 2.40mmol cuprous bromide in reaction flask 2 for 0.5h, stirred and refluxed in 4ml of water and 4mL40wt% hydrobromic acid mixture for 2h; reacted in reaction flask 1 at 50°C The...
specific Embodiment approach 2
[0035] 1. Take 15mmol 2,5-dibromoaniline, 36mmol p-ethoxyphenylboronic acid, 0.09mmol sodium carbonate and 0.6mmol tetrakistriphenylphosphine palladium and vacuum-dry for 0.5h, then mix in 75ml dioxane and 37.5ml water Stir and reflux at 80°C for 30 hours to stop the reaction; cool to room temperature, remove the solvent by rotary evaporation, add n-hexane to filter and wash; purify the crude product by column chromatography to obtain pure 2,5-bis(4'-ethoxy phenyl) aniline with a yield of 80%.
[0036] 2. Take 1.6mmol of the above-mentioned intermediate product and put it into reaction bottle 1, add 14.0ml of water and 0.26ml of 98% concentrated sulfuric acid mixture, stir and reflux at 60°C for 1.5h; then add 1ml of 1.76mol / L sodium nitrite at 0°C Solution, stirred and refluxed for 1h; vacuum-dried 2.40mmol cuprous bromide in reaction flask 2 for 0.5h, stirred and refluxed in 4ml of water and 4mL40wt% hydrobromic acid mixture for 2h; reacted in reaction flask 1 at 50°C The m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com