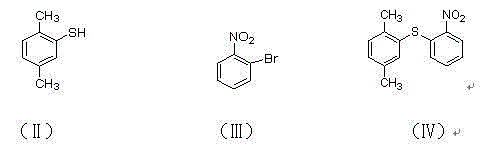Preparation method of vortioxetine hydrobromide
A technology of vortioxetine and ammonium formate, applied in the field of drug preparation, can solve the problems of increased production process and production cycle, low actual yield of reaction, unfavorable industrial production, etc., to save production time, avoid competitive side reactions, The effect of avoiding the risk of explosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
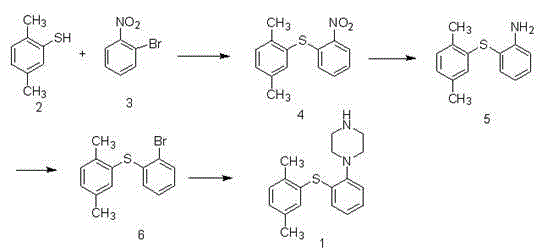
[0049] Preparation of compound (4) 2-(2,4-dimethylsulfanyl)nitrobenzene
[0050] In a 250ml reaction flask, dissolve 2,4-dimethylthiophenol (10.0g, 75mmol), o-bromonitrobenzene (18.2g, 90mmol), potassium carbonate (15.5g, 113mmol) in 150ml tetrahydrofuran, 65 Reaction at ℃ for 8 hours, the reaction was completed, lowered to room temperature, the reaction solution was concentrated, 250ml of water was added, stirred, and suction filtered to obtain 16.2g of a yellow-brown solid, the yield was 83.7%, ESI-MS: 260 (MH+), 1H-NMR ( DMSO-d6)δ8.1( m, 1H), 7. 6( m, 1H), 7.5( m, 2H), 7.4( s,1H), 7. 2(d, 1H), 7.0 (d, 1H), 2.5(s, 3H), 2.3(s, 3H).
[0051] Preparation of compound (5) 2-(2,4-dimethylphenylthio)aniline
[0052] In a 250ml reaction flask, dissolve compound (4) (13.0g, 50mmol) in 150mL methanol, add 1.0g 10% Pd / C, ammonium formate (12.6g, 200mmol), and continue to stir the reaction at 25°C After 1 h, suction filtration, the filtrate was concentrated under reduced pressure, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com