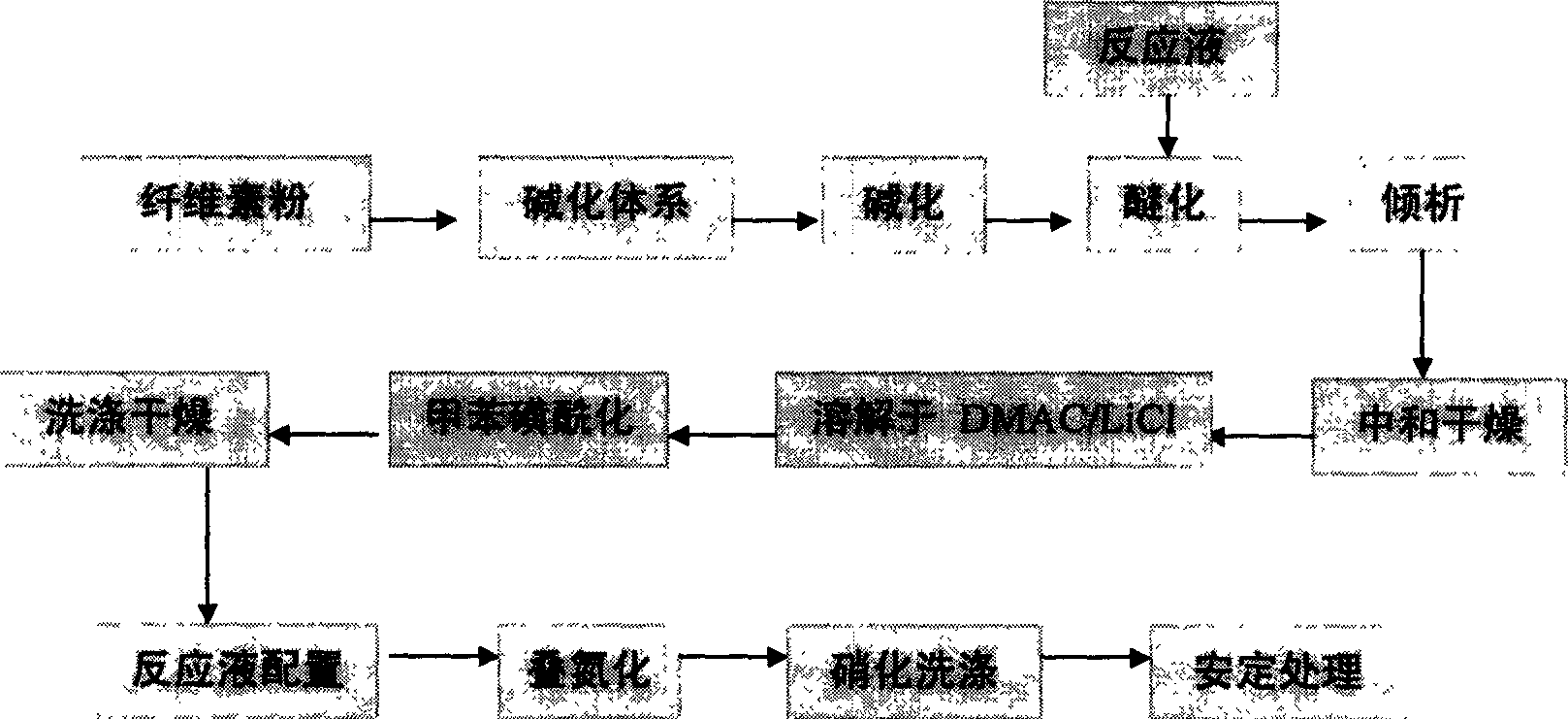
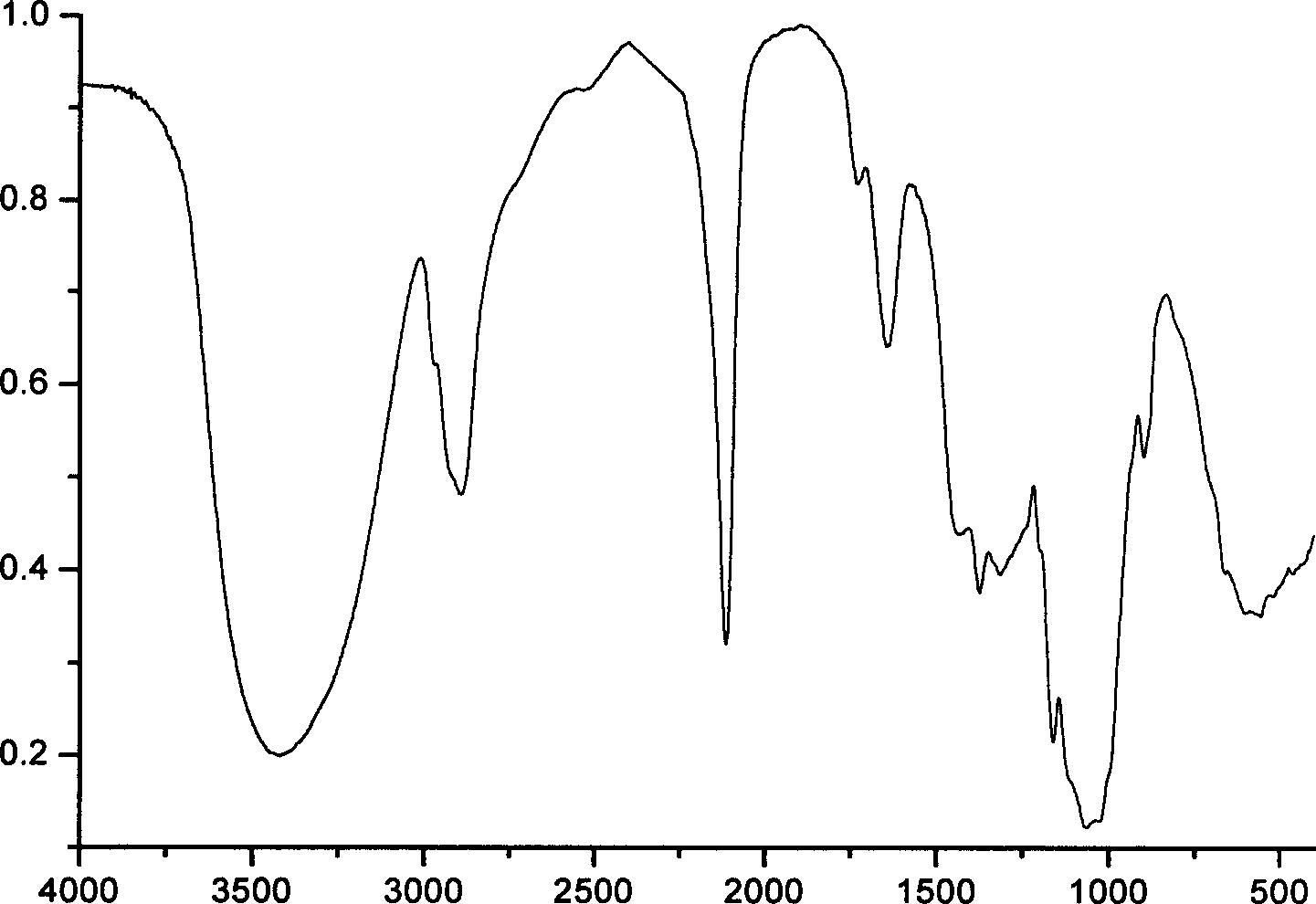
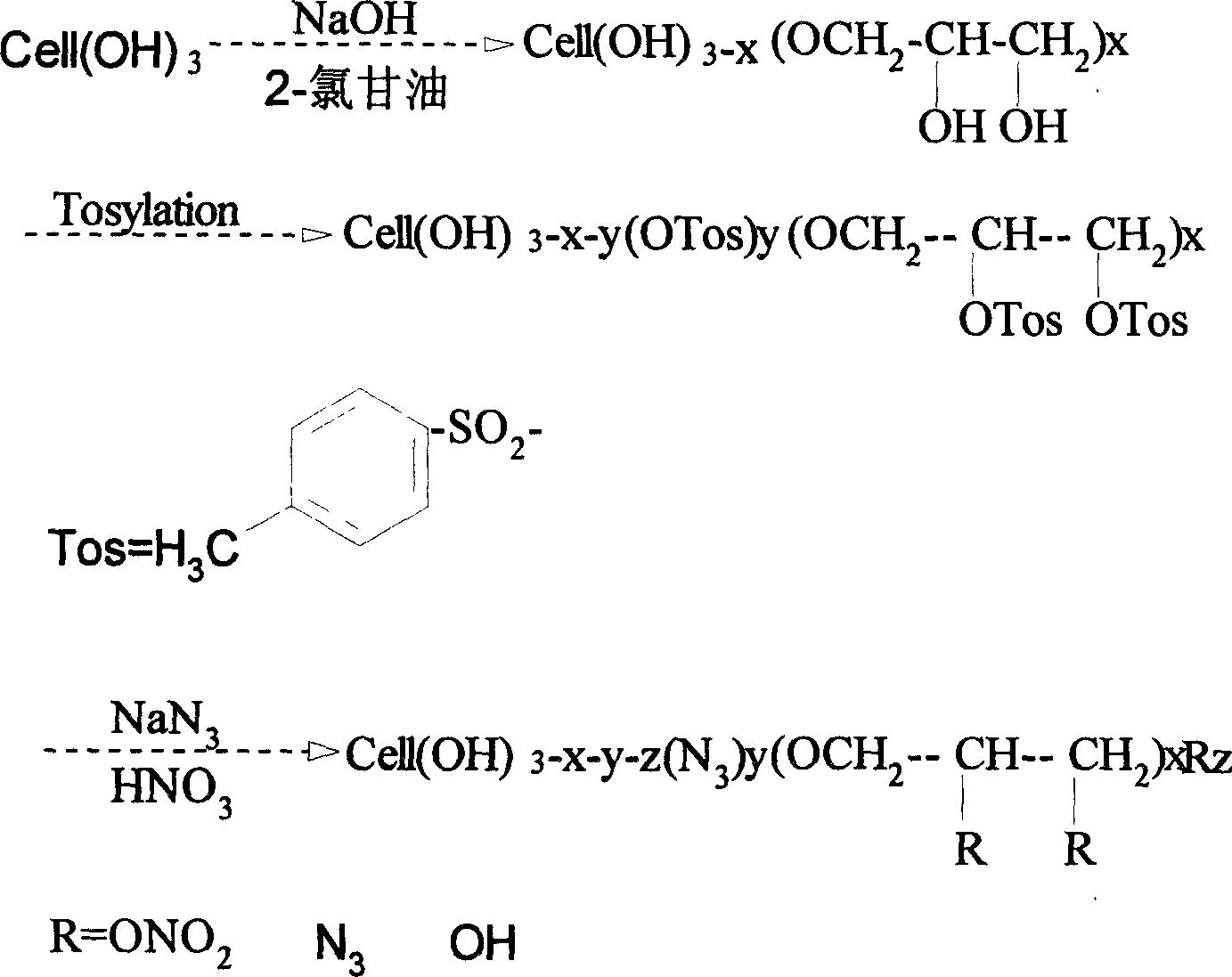Azido dihydroxypropyl cellulose nitrate preparation method and synthesis
A technology of dihydroxypropyl azide and dihydroxypropyl, applied in the field of polymer chemistry, can solve problems such as difficult to meet the overall requirements, poor thermoplasticity, difficult to remove halogens, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 15.11 parts of pulverized cotton cellulose with a degree of polymerization of 1000, 150.9 parts of isopropanol, and 25.0 parts of toluene were loaded into a reactor. NaOH solution (8.27 parts NaOH / 22.31 parts water) was added within 15 min and stirring was continued for 60 min. Add 15.63 parts of high-purity glycidyl alcohol dispersed in 18.31 parts of isopropanol and stir for 15 minutes to disperse. Gradually raise the temperature to 55°C and react for 1.5h. Under this condition, continue to add 45 parts of 50% NaOH solution to the reaction system within 15 minutes. After stirring for 10 minutes, add a dispersion of 15.51 parts of glycidol / 8.21 parts of isopropanol to the reaction system, and continue to stir for 15 minutes, then raise the temperature of the reaction to 68 ° C, and proceed for 1.5 hours. After cooling, use 85% isopropanol The aqueous solution is neutralized and washed twice. For the third time, use 80% acetone solution to dechlorinate. T...
Embodiment 2
[0021]Example 2: 14.96 parts of pulverized wood cellulose with a degree of polymerization of 900, 150.0 parts of isopropanol, and 25.2 parts of toluene were loaded into a reactor. NaOH solution (8.25 parts NaOH / 22.36 parts water) was added within 15 min and stirring was continued for 60 min. Add 15.05 parts of distilled glycidol dispersed in 25.66 parts of isopropanol at one time and stir for 15 minutes to disperse. Then the temperature was raised to 54°C and the reaction was carried out for 1.5h. Subsequently, the temperature of the reaction was raised to 65° C. for another 1.5 h, and after cooling, it was neutralized and washed twice with 85% isopropanol aqueous solution. For the third time, use 80% acetone solution to dechlorinate. Then dry at 65°C for 2 hours to obtain dihydroxypropyl cellulose ether as a white powder product. Dissolve 10.0 parts of dihydroxypropyl cellulose ether in dimethylformamide / 9% LiCl system, the solution concentration is controlled at 6%, and a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com