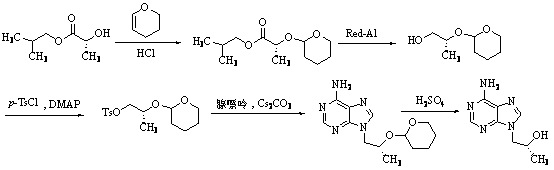
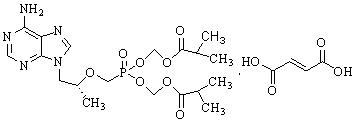
Method for synthesis of PMPA by combining biological technique and chemical technique
A chemical method, biological method, applied in the directions of biochemical equipment and methods, microorganism-based methods, chemical instruments and methods, etc., can solve the problems of unreachable and high production costs, and achieve simple post-processing and mild reaction conditions. , significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
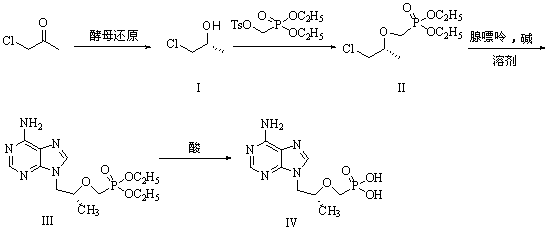
[0029] (1) Preparation of R-chloropropanol (I)
[0030] Add 10g Angel dry yeast to 150ml pH=5.91 buffer solution, place the reaction flask in a shaker at T=35℃, activate for 0.5 hours, add 0.9g chloroacetone, and sample with a centrifuge tube every 0.5h , 3mL each time; after sampling, centrifuge with a centrifuge, the speed of the centrifuge is: r=4000rpm, time t=20min; after centrifugation, take the supernatant liquid, evaporate it with a rotary evaporator, and add methanol to dissolve and let it stand still ; Do temperament analysis. When the reaction is complete, centrifuge and concentrate, add 50ml methanol to dissolve, filter and concentrate to obtain 0.65g of chloropropanol (I).
[0031] (2) Preparation of (R)-2-[bis-(isopropyl)-phosphonomethoxy]-propyl chloride (II)
[0032] Put 9.4g (100mmol) of compound (I) in a 200ML three-necked flask, add 100ML of anhydrous THF, under ice bath, add 2.88g (120mmol), 60% NaH in batches, stir for 0.5 hour, then slowly add 38.2 dropwise ...
Embodiment 2
[0038] (1) Preparation of R-chloropropanol (I)
[0039] Add 10g Angel dry yeast to 150ml pH=6.98 buffer solution, place the reaction flask in a shaker at T=35℃, activate for 0.5 hours, add 0.9g chloroacetone, and sample with a centrifuge tube every 0.5h , 3mL each time; after sampling, centrifuge with a centrifuge, the speed of the centrifuge is: r=4000rpm, time t=20min; after centrifugation, take the supernatant liquid, evaporate it with a rotary evaporator, and add methanol to dissolve and let it stand still ; Do temperament analysis. When the reaction is complete, centrifuge and concentrate, add 50ml methanol to dissolve, filter and concentrate to obtain 0.80g of chloropropanol (I).
[0040] (2) Preparation of (R)-2-[bis-(isopropyl)-phosphonomethoxy]-propyl chloride (II)
[0041] Put 9.4g (100mmol) of compound (I) in a 200ML three-necked flask, add 100ML of anhydrous THF, add 6.0g (150mmol) NaOH in batches under ice bath, stir for 0.5 hour, and then slowly drop 38.2g (120mmol)...
Embodiment 3
[0047] (1) Preparation of R-chloropropanol (I)
[0048] Add 10g Angel dry yeast to 150ml pH=8.04 buffer solution, place the reaction flask in a shaker at T=35℃, activate for 0.5 hours, add 0.9g chloroacetone, and sample with a centrifuge tube every 0.5h , 3mL each time; after sampling, centrifuge with a centrifuge, the speed of the centrifuge is: r=4000rpm, time t=20min; after centrifugation, take the supernatant liquid, evaporate it with a rotary evaporator, and add methanol to dissolve and let it stand still ; Do temperament analysis. After the reaction is complete, centrifugal concentration, add 50ml methanol to dissolve, filter and concentrate to obtain 0.89g of chloropropanol (I).
[0049] (2) Preparation of (R)-2-[bis-(isopropyl)-phosphonomethoxy]-propyl chloride (II)
[0050] Put 9.4g (100mmol) of compound (I) in a 200ML three-necked flask, add 100ML of anhydrous THF, add 6.0g (150mmol) NaOH in batches under ice bath, stir for 0.5 hour, and then slowly drop 38.2g (120mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com