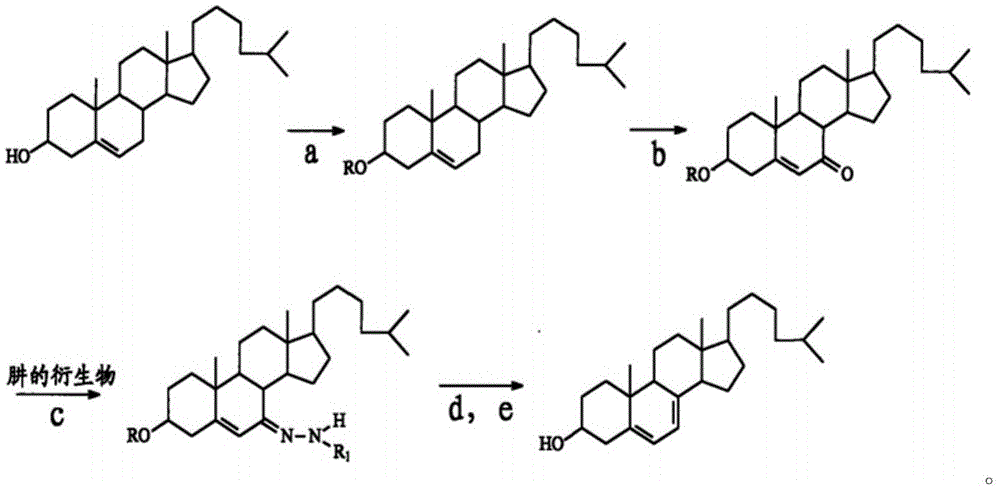
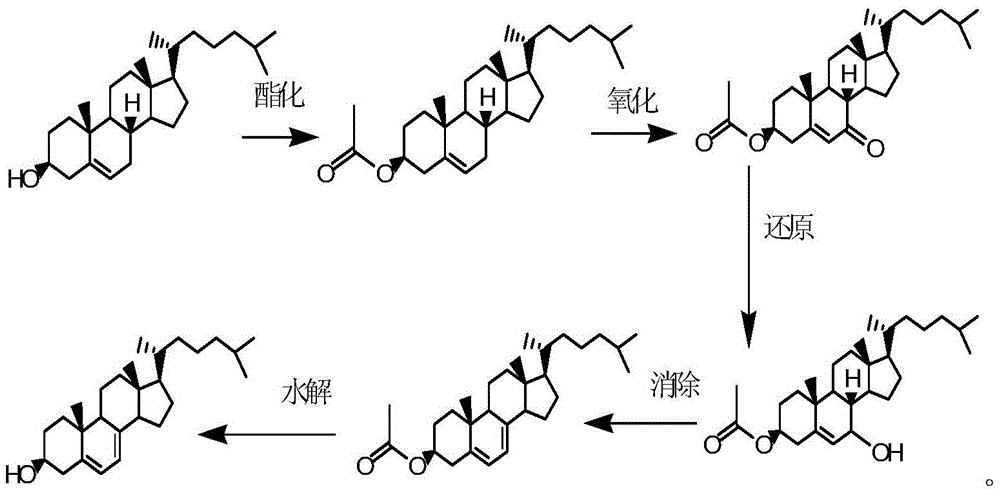
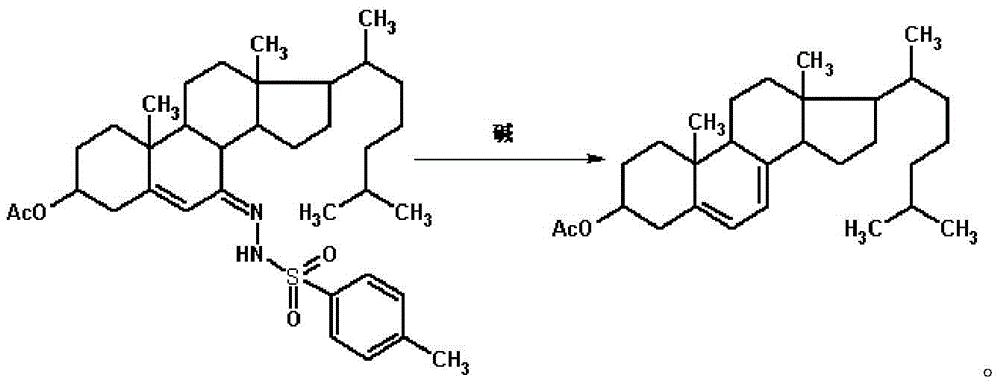
Method for preparing 7-dehydrogenized cholesteryl ester from 7-tosylhydrazones-3-cholesteryl ester
A technology of p-toluenesulfonylhydrazone and dehydrocholesteryl ester, which is applied in the field of organic chemical synthesis, can solve the problems of high production cost, reaction selectivity and low product yield, achieve short reaction time, significantly reduce production cost, and improve The effect of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Swell 10 g of chloromethylated polystyrene resin (2% divinylbenzene cross-linked, 55 mequivCl per gram of resin, 80-100 mesh) in anhydrous tetrahydrofuran for 1 h, add anhydrous potassium carbonate 3.72 g (27.6 mmol) and 9.04g of 4'-aminobenzo-18-crown ether-6 (27.6mmol), and in 80ml of anhydrous tetrahydrofuran solution, stirred and refluxed under nitrogen flow, filtered the resin after cooling, washed with acetone, water and acetone successively, and then Extracted with acetone and dried, a light yellow resin can be obtained. The latter is refluxed with excess sodium methoxide solution until it does not contain Cl, and then filtered, extracted with acetone and dried in vacuum to obtain a polystyrene resin-supported 4'-aminobenzo-18-crown-6 catalyst.
[0039]
Embodiment 2
[0041]Swell 10g of chloroethylated polystyrene resin (2% divinylbenzene crosslinked, 55 mequivCl per gram of resin, 80-100 mesh) in anhydrous tetrahydrofuran for 1 hour, add anhydrous potassium carbonate 3.72g (27.6mmol) and 7.82g of 4'-aminobenzo-15-crown-5 (27.6mmol), and in 80ml of anhydrous tetrahydrofuran solution, stirred and refluxed under nitrogen flow, filtered the resin after cooling, washed with acetone, water and acetone successively, and then Extracted with acetone and dried, a light yellow resin can be obtained. The latter is refluxed with excess sodium methoxide solution until it does not contain Cl, and then the product is obtained after filtration, extraction with acetone and vacuum drying.
Embodiment 3
[0043] Swell 10 g of chloromethylated polystyrene resin (2% divinylbenzene cross-linked, 55 mequivCl per gram of resin, 80-100 mesh) in anhydrous tetrahydrofuran for 1 h, add anhydrous potassium carbonate 3.72 g (27.6 mmol) and 10.86g4,4'-diaminodibenzo-18-crown-6 (27.6mmol). And in 80ml of anhydrous tetrahydrofuran solution, stirred and refluxed under a nitrogen stream, the resin was cooled and filtered, followed by acetone, water and acetone After washing, extraction with acetone and drying, a light yellow resin can be obtained. The latter is refluxed with excess sodium methoxide solution until it does not contain Cl, and then the product is obtained after filtration, extraction with acetone and vacuum drying.
[0044] Replace the crown ethers used above, such as 8-crown-6, benzo-18-crown-6, dibenzo-18-crown-6, 15-crown-5, benzo15-crown-5, 12 -crown-4, dicyclohexane-18-crown-6, dibenzo-15-crown-5, etc., can obtain a variety of different polystyrene resin supported crown eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com