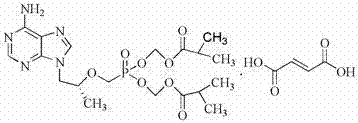
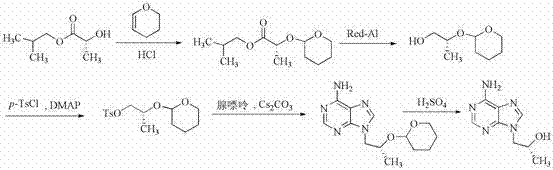
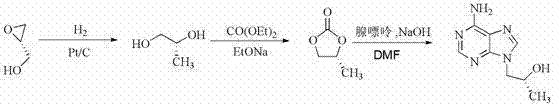Synthetic process of tenofovir
A synthesis process and reaction bottle technology, which is applied in the field of synthesis process for the preparation of anti-AIDS and anti-hepatitis B drug Tenofovir, can solve the problems of unreachable and high production costs, achieve mild reaction conditions, avoid reaction conditions, Significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 (1) Preparation of R-1,2-propanediol (I)
[0034] Take a 250 mL three-neck flask, add sodium borohydride (5.7 g, 0.15 mol), methyl lactate (10.4 g, 0.10 mol) and toluene (60 mL), stir at room temperature for 2 hours, then add 5 mL of methanol (note: the reaction violently emits Add 5 mL of methanol 10 minutes later, carefully add 5 mL of methanol for the third time after 10 minutes to stop the reaction, cool to room temperature and adjust the pH value to neutral with 5 mol / L hydrochloric acid (Note: the gas is also released violently, it needs to be added carefully), the system produces a large amount of insoluble matter, the insoluble matter is filtered out, and the diol product is obtained after distilling off the solvent. It was directly used in the next reaction without purification.
[0035] (2) Preparation of R-propylene carbonate (II)
[0036] Take a 50 mL three-neck flask with a spherical condenser, add a stirring magnet, and then measure 2 mL of...
Embodiment 2
[0043] Example 2 (1) Preparation of R-1,2-propanediol (I)
[0044] Take a 250 mL three-neck flask, add lithium aluminum hydride (5.7 g, 0.15 mol) and THF (100 mL), add methyl lactate (10.4 g, 0.10 mol) in batches, stir at room temperature for 2 hours, then slowly add 50 mL THF dropwise The mixture with water was cooled to room temperature and then filtered, the filter cake was washed several times with THF, the filtrates were combined, and the diol product was obtained after distilling off the solvent. It was directly used in the next reaction without purification.
[0045] (2) Preparation of R-propylene carbonate (II)
[0046] Take a 50 mL three-neck flask with a spherical condenser, add a stirring magnet, and then measure 2 mL of absolute ethanol into the bottle. Weigh sodium methoxide (0.50 g, 0.009 mol), add diol (6.61 g, 89.7 mmol), heat to reflux for 30 minutes, slowly drop diethyl carbonate (12.98 g, 0.11 mol), control the rate of addition, the system releases ho...
Embodiment 3
[0053] Example 3 (1) Preparation of R-1,2-propanediol (I)
[0054] Take a 250 mL three-neck flask, add sodium borohydride (7.6 g, 0.20 mol), tert-butyl lactate (14.6 g, 0.10 mol) and cyclohexane (100 mL), stir at room temperature for 2 hours, then add 15 mL of methanol to stop the reaction , after cooling to room temperature, adjust the pH value to neutral with 5 mol / L hydrochloric acid, the system produces a large amount of insoluble matter, filter out the insoluble matter, and diol product is obtained after distilling off the solvent. It was directly used in the next reaction without purification.
[0055] (2) Preparation of R-propylene carbonate (II)
[0056] Take a 50 mL three-neck flask with a spherical condenser, add a stirring magnet, and then measure 2 mL of absolute ethanol into the bottle. Weigh sodium methoxide (0.50 g, 0.009 mol), add diol (6.61 g, 89.7 mmol), heat to reflux for 30 minutes, slowly drop diethyl carbonate (12.98 g, 0.11 mol), control the rate o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com