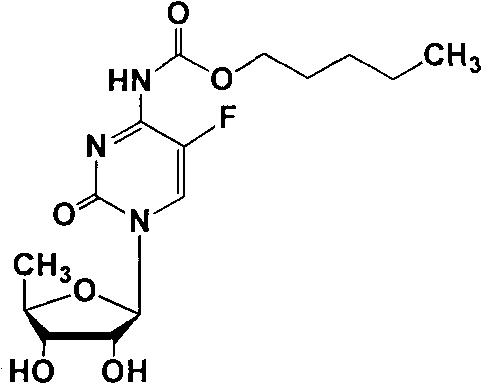
Preparation methods of capecitabine and intermediate thereof
A technology of capecitabine and methyl, which is applied in the field of preparation of capecitabine and its intermediates, can solve the problems affecting the yield of products, and achieve the effects of avoiding by-products, reducing pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
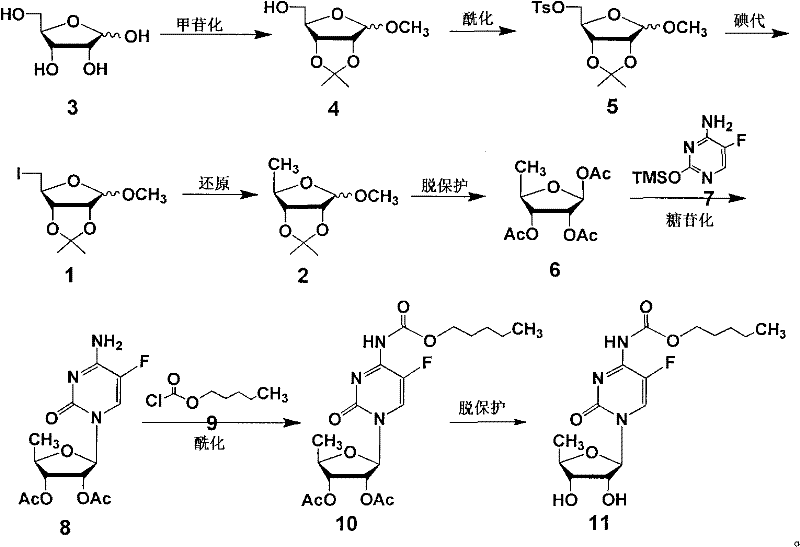
[0036] Preparation of 1-O-methyl-2,3-O-isopropylidene-D-ribofuranose (4):
[0037] D-ribose (3) (20g, 133mmol) was dissolved in methanol (80ml) and acetone (120ml), concentrated hydrochloric acid (2ml) was added, the mixture was refluxed for 3h, allowed to cool, neutralized with solid sodium bicarbonate The pH of the solution was 6-7, the solvent was evaporated under reduced pressure, water (60ml) and ethyl acetate (100ml) were added, the organic phase was separated, the aqueous phase was extracted with ethyl acetate, the organic phase was washed and dried, filtered, and evaporated to dryness to obtain an oily Material 4 (23.6g, 86.8%) can be directly used in the next reaction.
Embodiment 2
[0039] Preparation of 1-O-methyl-2,3-O-isopropylidene-5-O-p-methylbenzenesulfonyl-D-ribofuranose (5):
[0040] Dissolve the oil 4 (47g, 0.23mol) in dichloromethane (200ml), add triethylamine (56ml), cool to 0-5°C, add p-toluenesulfonyl chloride (62.4g) six times, Each interval is about 10min. After the addition is completed, continue to keep at 5°C, stir for 3h, wash with water (50ml), 1mol / L hydrochloric acid (25ml), saturated aqueous sodium bicarbonate solution (25ml) and water (50ml) successively, evaporate to dryness, and add Ice water, stirred, filtered, washed with water, and dried to obtain solid 5 (60g, 72.7%), mp 80-81°C.
Embodiment 3
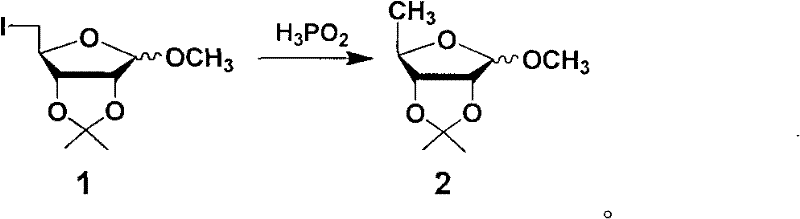
[0042] Preparation of 1-O-methyl-2,3-O-isopropylidene-5-deoxy-5-iodo-D-ribofuranose (1):
[0043] Compound 5 (10g, 31.8mmol) was dissolved in acetonitrile (104ml), sodium iodide (12.5g) was added, and the reaction was refluxed for 12h. Let cool, filter, distill off the solvent, add isopropyl ether to stir, filter, wash with isopropyl ether, and evaporate the filtrate to dryness under reduced pressure to give yellow oil 1 (8.5 g, 100%). No need to deal with, directly put into the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com