<18>F-labeled aggregation-induced emission (AIE) fluorescent/positron emission tomography (PET) dual-mode probe, and preparation method and application thereof
A technology of 18F-TPE-TEG and 18F, which is applied in the field of AIE fluorescence/PET dual-mode probe compound and its preparation, can solve the problems of low spatial resolution, inability to accurately locate the lesion, difficulty in popularization, etc., and achieve high spatial resolution Resolution and sensitivity, strong ability to resist photobleaching, and good luminescence stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
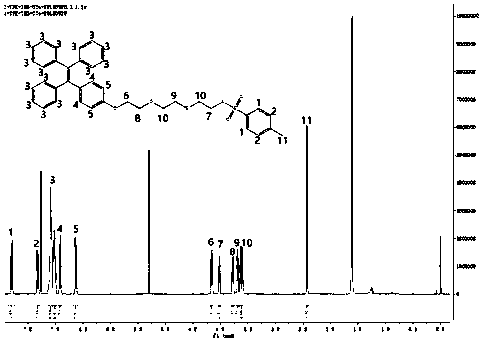
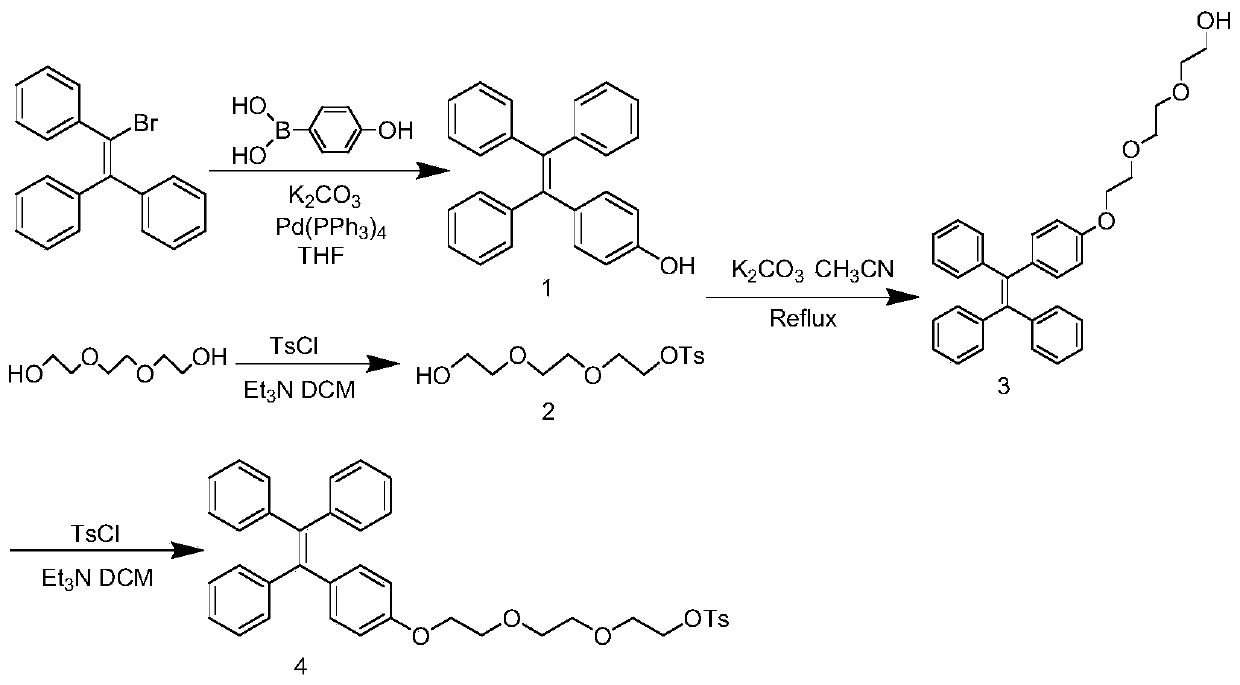
[0043] The synthetic route of the labeled precursor of the present invention is as follows: figure 1 Shown, below in conjunction with a specific example, the present invention will be further described:
[0044] 4-Hydroxytetraphenylethylene (1)
[0045] 4.00g (12.0mmol) 2-bromo-1,1,2-triphenylethylene, 2.00g (14.3mmol) 4-hydroxyphenylboronic acid, 0.1597g (0.5mmol) tetrabutylammonium bromide, 7.25mL 2M K 2 CO 3 Solution, 0.0293g (0.025mmol) tetrakistriphenylphosphine palladium was added into 25mL tetrahydrofuran, reflux reaction at 79°C for 14h, and cooled. After filtration, the solvent was removed in vacuo, and the solid was subjected to column chromatography to obtain 2.0 g of a white solid product (yield: 49.6%).
[0046] 8-p-Toluenesulfonyloxy-3,6-dioxoctanol (2)
[0047] 12.0g (80mmol) triethylene glycol, 5.6mL (40mmol) triethylamine, 3.82g (20mmol) p-toluenesulfonyl chloride were dissolved in 100mL dichloromethane, stirred at room temperature for 14h, added 80mL 1M KHS...
Embodiment 2
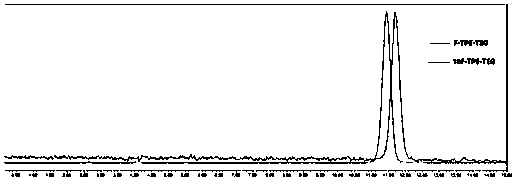
[0053] Labeled precursors of the present invention 19 F-labeled standard product F-TPE-TEG synthesis route such as figure 2 As shown, combined with examples to illustrate as follows:
[0054] 8-Fluoro-3,6-dioxoctyltetrastyryl ether (5)
[0055] 0.1g (0.21mmol) of 8-tetrastyryloxy-3,6-dioxoctanol and 0.1088g (0.64mmol) of diethylaminosulfur trifluoride were dissolved in 1mL of dichloromethane and reacted at room temperature for 35h. Add 10 mL of dichloromethane, wash with 8 mL of water, then wash with 8 mL of saturated potassium carbonate solution, and finally wash with 8 mL of saturated sodium chloride solution, and dry over anhydrous sodium sulfate. The solvent was removed in vacuo, and the solid was separated by column chromatography to obtain 0.0268 g (yield: 26.7%) of a yellow oily liquid, which was the standard product F-TPE-TEG.
Synthetic example
[0057] of the present invention 18 F-labeled AIE fluorescent / PET dual-modal probe 18 The synthesis example of F-TPE-TEG is as follows:
[0058] Bombardment of oxygen-enriched water H by cyclotron 2 18 O, get 18 F ion, adsorbed by anion exchange column (QMA) 18 F ion, with 1mL containing 0.8mL acetonitrile, 0.2mL H 2 O, 4 mg K 2 CO 3 and 10mg K2.2.2 eluent will 18 F ions were eluted from the QMA column and transferred to the reaction flask, and 1 mL (7 mmol / L, dissolved in acetonitrile) of the labeled precursor compound 4 prepared in Example 1 was added, and the reaction was heated at 100°C for 15 min, namely get compound 18 F-TPE-TEG.
[0059] Add 2.5 mL of acetonitrile to the reaction solution for dilution, take a small amount of radioactive solution for dilution, and detect the labeling of the precursor by radioactive high-performance liquid chromatography. Wash the pipeline with 1 mL of acetonitrile, purify it through a semi-preparative C18 column, and use a vial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com