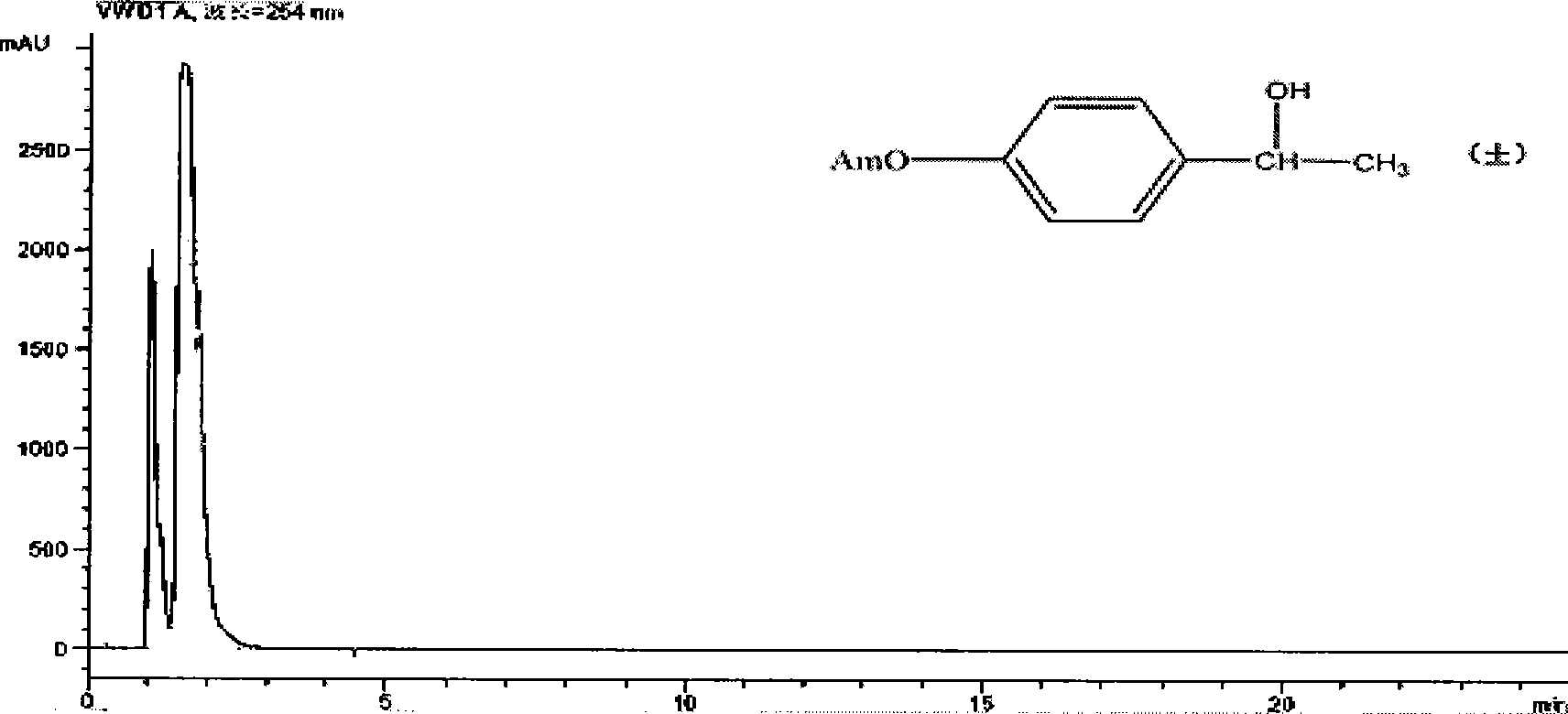
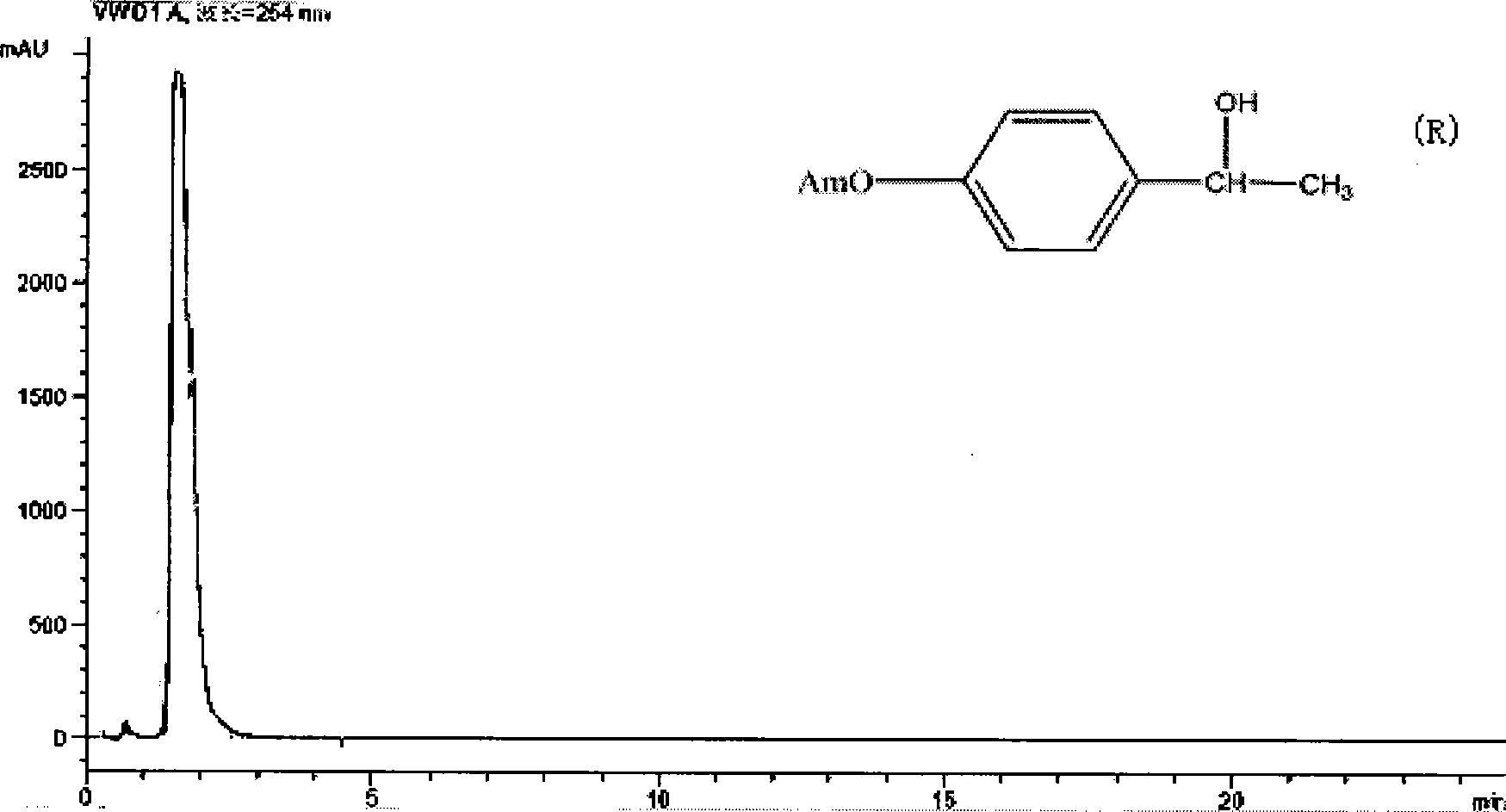
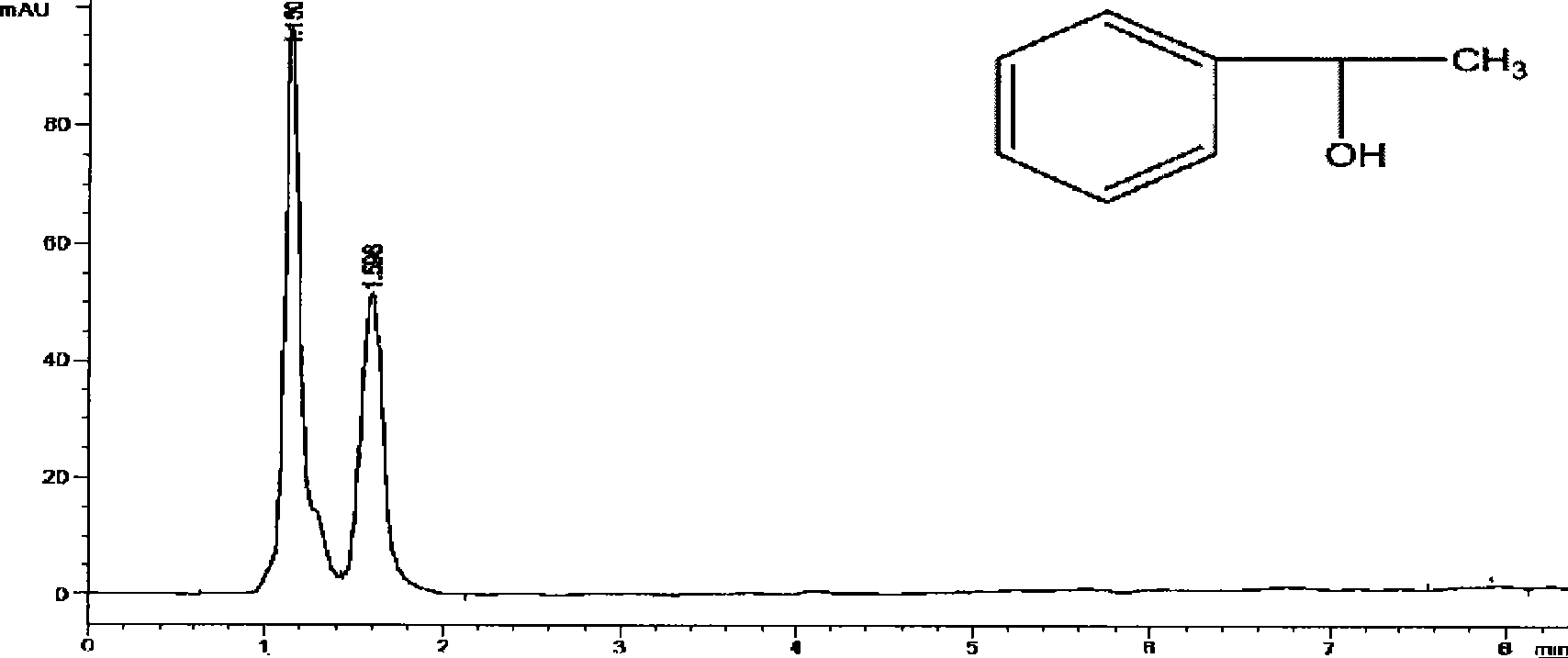
Silica gel bonded double-chirality active center chromatogram filler, preparation method and use thereof
A technology of active center and chromatographic packing, which is applied in the field of preparation of microcrystalline cellulose derivatives bichiral high performance liquid chromatography chiral chromatographic packing, which can solve the problem of derivatization reagents with few chiral centers and bonded chiral stationary phases. Few, no bonded dual-chiral chromatography packing reported, to achieve short separation time, high column efficiency, good permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Silica gel activation: According to the volume mass ratio of hydrochloric acid to silica gel 2ml / g, prepare a mixture of 20% hydrochloric acid and silica gel with a pore size of 30nm in volume percentage, heat at 90°C for reflux for 6 hours, suction filter, and wash with deionized water to medium properties, baked at 105°C for 4 hours, and vacuum-dried at 50°C for 6 hours to obtain activated silica gel for use;
[0041] (2) Microcrystalline cellulose 6-hydroxyl protection: 1.6g microcrystalline cellulose, 7.0g triphenylchloromethane, then add 50ml of anhydrous pyridine to react in a 100ml three-necked flask, at 80 ° C, mechanically stirred, Pass N 2 Reflux for 24 hours under certain reaction conditions, after the reaction is completed, filter and wash with methanol to obtain 6-hydroxyl-protected microcrystalline cellulose;
[0042] (3) Carboxylic acid chlorination: 10mlDL-2-phenylpropionic acid and 6ml sulfur oxychloride at 50°C, mechanically stirred, and N 2 Refl...
Embodiment 2
[0046](1) Silica gel activation: According to the mass ratio of hydrochloric acid to silica gel volume mass ratio 3ml / g, prepare a mixture of 20% hydrochloric acid by volume percentage and 100nm pore size of silica gel, heat at 93°C for reflux for 6 hours, suction filter and wash with deionized water To neutrality, bake at 105°C for 4 hours, and vacuum-dry at 50°C for 6 hours to obtain activated silica gel for use;
[0047] (2) Microcrystalline cellulose 6-hydroxyl protection: microcrystalline cellulose 2.0g, triphenylchloromethane 8.0g, with 50ml anhydrous pyridine as solvent, at 90 ℃, mechanical stirring, logical N 2 Reflux for 24 hours under certain reaction conditions, after the reaction is completed, filter and wash with methanol to obtain 6-hydroxyl-protected microcrystalline cellulose;
[0048] (3) Carboxylic acid chlorination: 12mlDL-2-phenylpropionic acid and 7.5ml thionyl chloride were placed at 50°C, mechanically stirred, and N 2 Reflux under reaction conditions fo...
Embodiment 3
[0052] (1) Silica gel activation: According to the volume mass ratio of hydrochloric acid to silica gel 2-6ml / g, prepare a mixture of 20% hydrochloric acid by volume and silica gel with a pore size of 200nm, heat at 90-100°C and reflux for 6 hours, filter with deionization Wash with water until neutral, bake at 105°C for 4 hours, and vacuum-dry at 50°C for 6 hours to obtain activated silica gel for use;
[0053] (2) Protection of the 6-position hydroxyl group of microcrystalline cellulose: 3.0 g of microcrystalline cellulose, 10.5 g of triphenylchloromethane and 50 ml of anhydrous pyridine, at 90 ° C, mechanically stirred, and nitrogen 2 Reflux for 24 hours under certain reaction conditions, after the reaction is completed, use methanol suction filtration and washing to obtain 6-hydroxyl-protected microcrystalline cellulose;
[0054] (3) Carboxylic acid chlorination: 12mlDL-2-phenylpropionic acid and 7ml thionyl chloride at 50°C, mechanically stirred, and N 2 Reflux under rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com