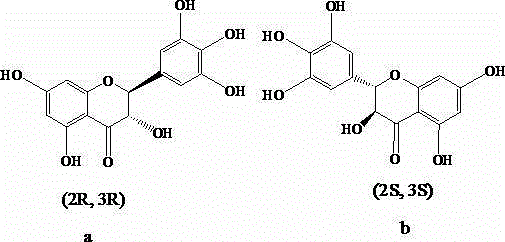
Chiral chromatographic separation and analysis method of dihydromyricetin enantiomer
A technique for separation and analysis of dihydromyricetin, applied in the field of chiral chromatographic separation, can solve the problems of physiological activity, toxicity and clinical efficacy, difficult elution and separation of mobile phases, etc., and achieve good chiral separation and analytical separation. Fast speed and improved peak broadening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 in Enantiopak TM Chiral Separation of Dihydromyricetin Enantiomers on AD Column
[0088] Firstly, the dihydromyricetin sample (purity>98%) was dissolved in the mobile phase to prepare a concentration of 0.50 mg·mL -1 solution; on the Agilent 1200 Series HPLC, using Enantiopak TM AD chromatographic column (150×4.6mm), n-hexane: trifluoroacetic acid: ethanol=80:0.1:20 (v / v / v) as the mobile phase, the flow rate is 1.0mL min -1 , the column temperature was 25° C. and the detection wavelength was 290 nm, and the above sample solution was chromatographically separated.
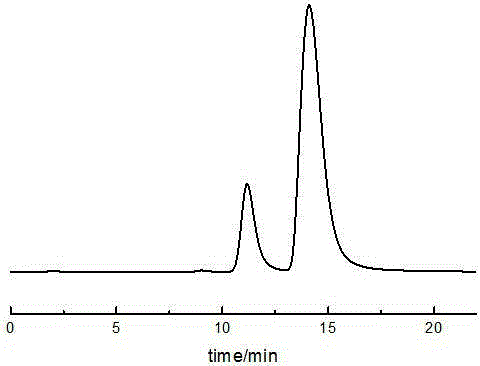
[0089] Such as figure 1 As shown, two chromatographic peaks appeared in the dihydromyricetin sample at 11.26min and 14.25min respectively, and the resolution was 1.95.
Embodiment 2
[0090] Example 2 in Enantiopak TM Chiral Separation of Dihydromyricetin Samples on AD Chromatographic Column
[0091] Firstly, dihydromyricetin sample (purity>98%) was dissolved in mobile phase to prepare a concentration of 10 mg·mL -1 solution; on the Agilent 1200 Series HPLC, using Enantiopak TM AD chromatographic column (150×4.6mm), n-hexane: trifluoroacetic acid: ethanol=80:0.1:20 (v / v / v) as the mobile phase, the flow rate is 1.0mL min -1 The dihydromyricetin sample was chromatographically separated at a column temperature of 25°C, and the separation of the sample was monitored by a CHIRALYSER-MP polarimetric detector.
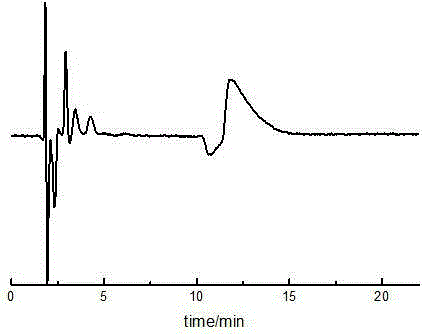
[0092] Such as figure 2 As shown, two chromatographic peaks occurred in the sample at 11.14min and 12.25min, and the peak time was close to that of Example 1. The optical rotation of the isomer eluting first is negative, and the isomer eluting later is positive. The detection of the sample by an optical rotation detector shows that the dihydromyricet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com