Preparation method of metallic organic macrocyclic crystalline-state material for splitting chiral amine
A metal-organic and chiral amine technology, which is applied in the field of preparation of metal-organic macrocyclic crystalline materials, can solve the problems of complex filling, expensive derivative-type chiral gas chromatography columns, cumbersome synthesis, etc., and achieve low reaction temperature, The effect of stable structure and simple synthesis operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
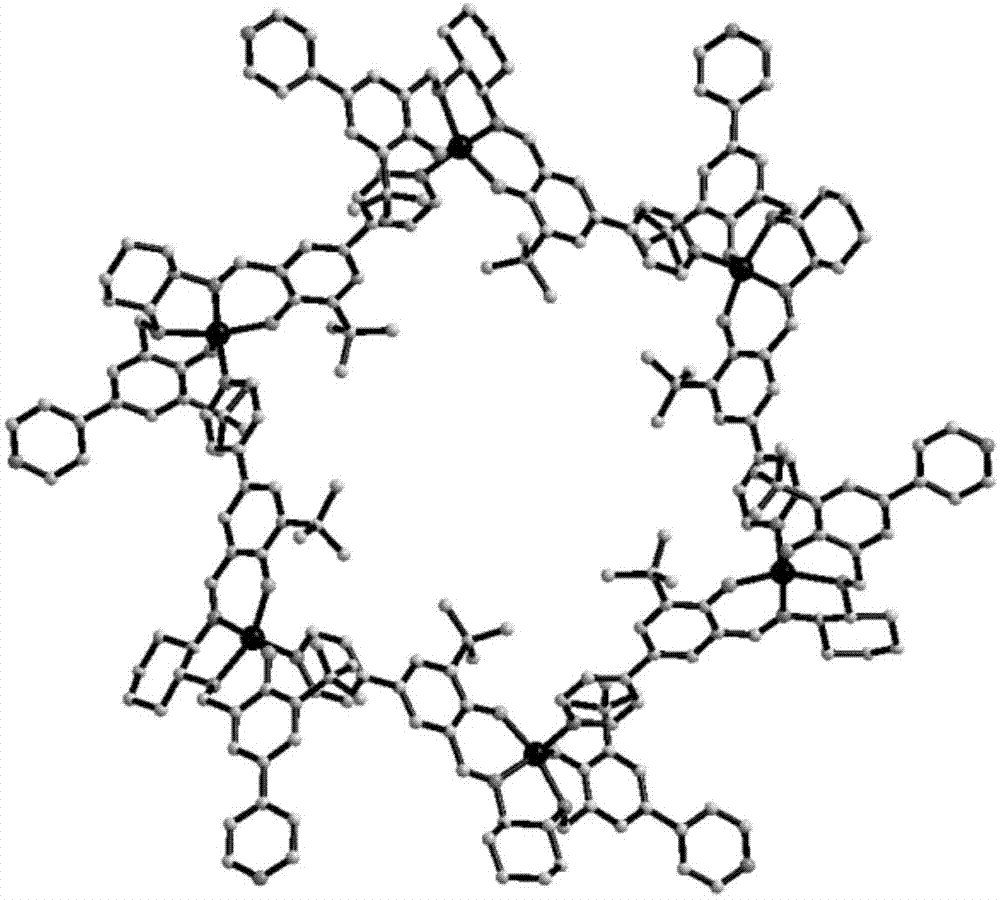
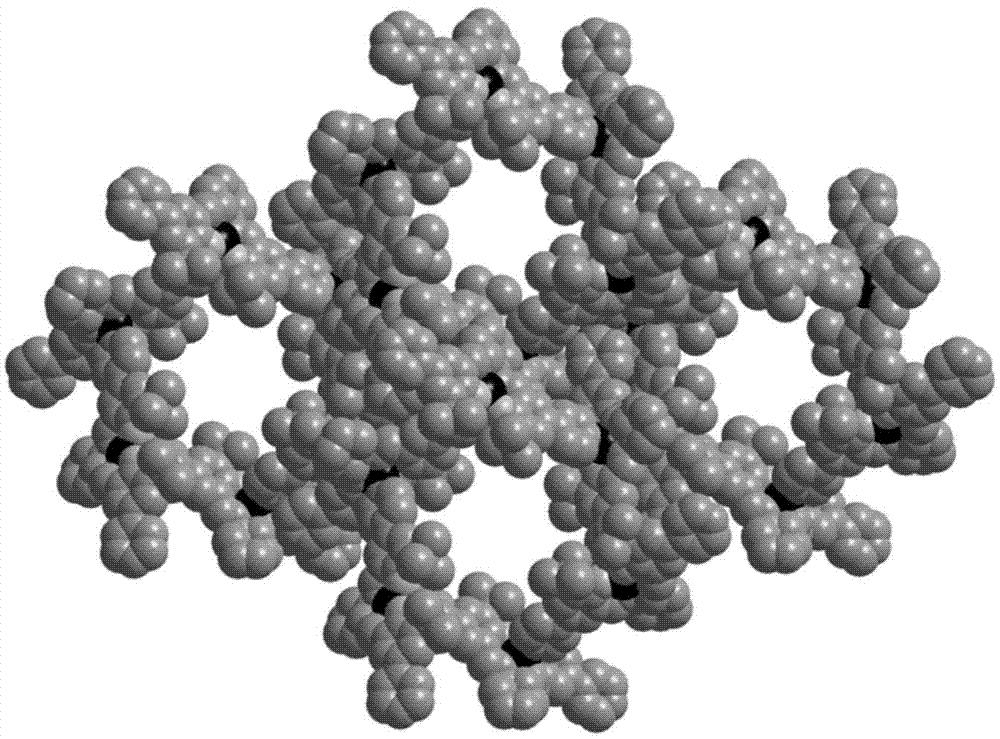
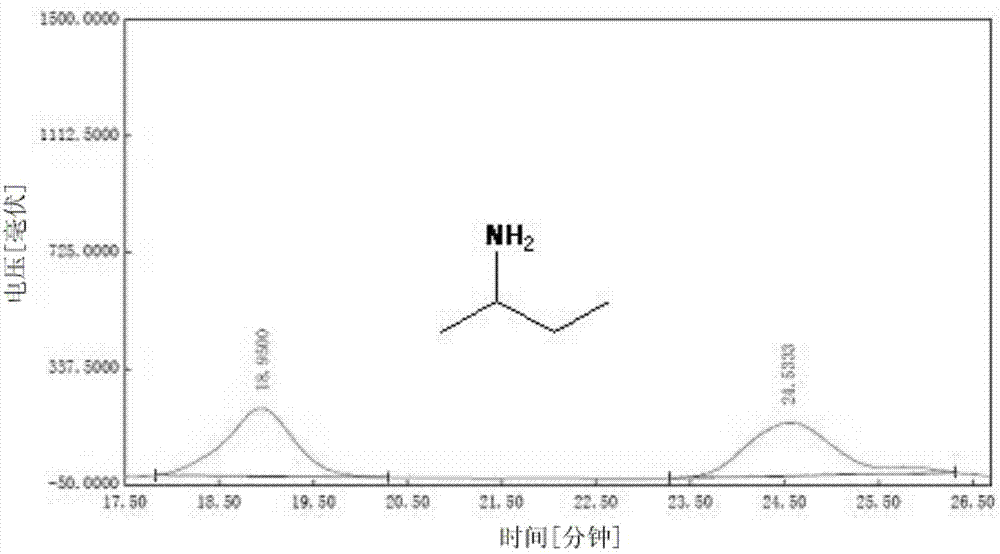
[0033] A method for preparing a metal organic macrocyclic crystalline material for resolving chiral amines, the method first synthesizes a chiral ligand (1R, 2R)-H 2 L, the chiral ligand (1R, 2R)-H 2 L is R type, specifically (1R, 2R)-N-2-hydroxy-3-tert-butyl-5-(4-pyridine)benzylidene-N'-2-hydroxy-3-tert-butyl- 5-(4-pyridine) benzyl-cyclohexanediamine, and then prepare metal-organic macrocyclic crystalline materials for splitting chiral amines by solvothermal method, comprising the following steps:
[0034] (1) Chiral ligand (1R, 2R)-H 2 Preparation of L:
[0035]① Add 151mg (1R, 2R)-cyclohexanediamine unilateral hydrochloride into 20ml of anhydrous methanol, dissolve, and prepare a solution with a mass concentration of 7.55mg / ml, and then slowly dropwise add a mass concentration of 12.75mg / ml 20ml of 3-tert-butyl-2-hydroxy-5-(4-pyridine)benzaldehyde in anhydrous methanol solution, heated to reflux at 70°C for 6h, spin-dried, washed with anhydrous ether, filtered, and dried...
Embodiment 2
[0076] A method for preparing a metal organic macrocyclic crystalline material for resolving chiral amines, the method first synthesizes a chiral ligand (1R, 2R)-H 2 L, the chiral ligand (1R, 2R)-H 2 L is R type, specifically (1R, 2R)-N-2-hydroxy-3-tert-butyl-5-(4-pyridine)benzylidene-N'-2-hydroxy-3-tert-butyl- 5-(4-pyridine) benzyl-cyclohexanediamine, and then prepare metal-organic macrocyclic crystalline materials for splitting chiral amines by solvothermal method, comprising the following steps:
[0077] (1) Chiral ligand (1R, 2R)-H 2 Preparation of L:
[0078] ① Add (1R, 2R)-cyclohexanediamine unilateral hydrochloride into 20ml of anhydrous methanol, dissolve, and prepare a solution with a mass concentration of 7.2mg / ml, then slowly add 16ml of it with a mass concentration of 12.5mg / ml ml of 3-tert-butyl-2-hydroxy-5-(4-pyridine)benzaldehyde in anhydrous methanol solution, heated to reflux at 75°C for 7h, spin-dried, washed with anhydrous ether, filtered, and dried to ob...
Embodiment 3
[0084] A method for preparing a metal organic macrocyclic crystalline material for resolving chiral amines, the method first synthesizes a chiral ligand (1R, 2R)-H 2 L, the chiral ligand (1R, 2R)-H 2 L is R type, specifically (1R, 2R)-N-2-hydroxy-3-tert-butyl-5-(4-pyridine)benzylidene-N'-2-hydroxy-3-tert-butyl- 5-(4-pyridine) benzyl-cyclohexanediamine, and then prepare metal-organic macrocyclic crystalline materials for splitting chiral amines by solvothermal method, comprising the following steps:
[0085] (1) Chiral ligand (1R, 2R)-H 2 Preparation of L:
[0086] ① Add (1R, 2R)-cyclohexanediamine unilateral hydrochloride into 18ml of anhydrous methanol, dissolve, and prepare a solution with a mass concentration of 8.5mg / ml, and then slowly add 20ml of it with a mass concentration of 13.5mg / ml ml of 3-tert-butyl-2-hydroxy-5-(4-pyridine)benzaldehyde in anhydrous methanol solution, heated to reflux at 80°C for 8 hours, spin-dried, washed with anhydrous ether, filtered, and dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com