Chiral chromatographic separation analysis method of dihydroquercetin enantiomer
A dihydroquercetin, separation and analysis technology, applied in material separation, analysis materials, measuring devices, etc., can solve problems such as differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of chiral chromatographic separation analysis method of dihydroquercetin enantiomer is as follows:
[0027] (1) Instruments and conditions: High performance liquid chromatography: Agilent 1200 series liquid chromatography
[0028] Chromatographic column: the specification is 250×4.6mm; the column is filled with cellulose-tris(4-methylbenzoate) chiral stationary phase produced by Guangzhou Yanchuang Biotechnology Development Co., Ltd., namely Y2.
[0029] Mobile phase: n-hexane: trifluoroacetic acid: ethanol = 80:0.1:20 (V / V / V)
[0030] Flow rate: 1.0mL / min
[0031] Column temperature: 25°C
[0032] Injection volume: 10μL
[0033] Detection wavelength: 290mm
[0034] (2) Implementation
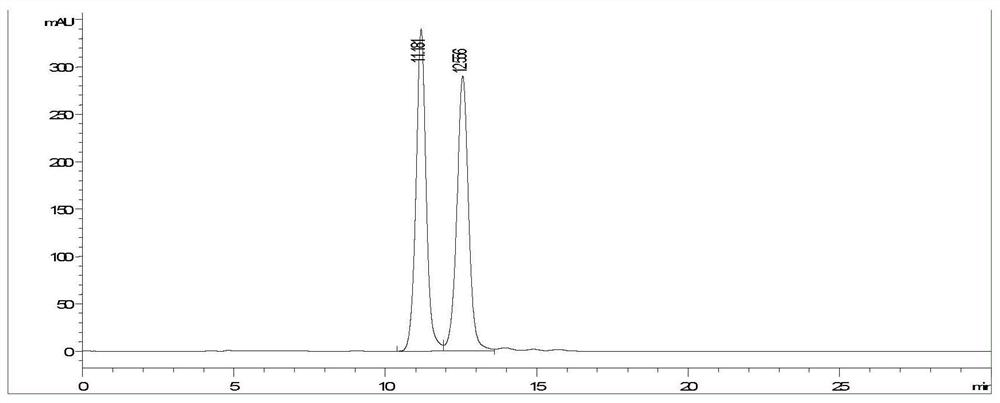
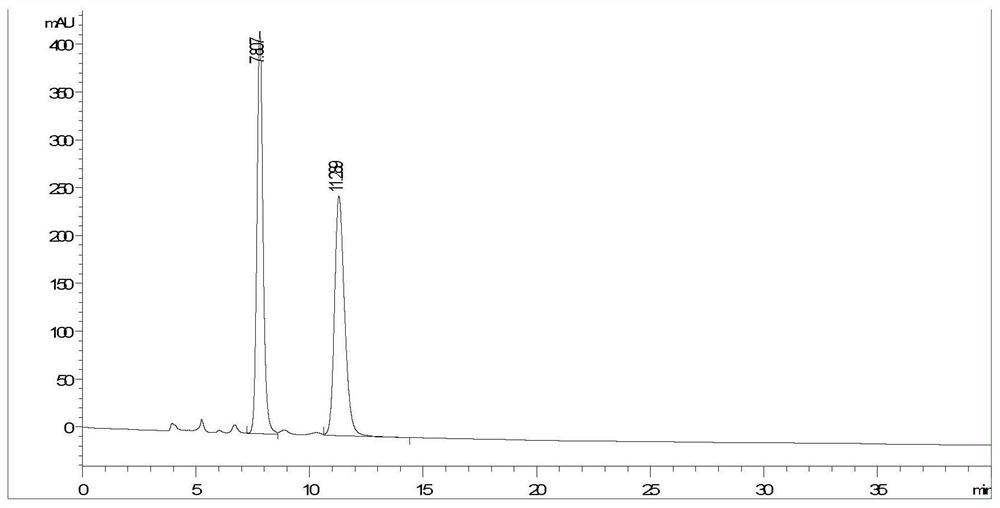
[0035] Dihydroquercetin (purity is 99%) is formulated into 0.5mg / mL solution with ethanol, on Agilent 1200 series high-performance liquid chromatograph, adopt Y2 chromatographic column (250×4.6mm), the mobile phase is n-hexane:trifluoroacetic acid:ethanol=80:0.1:20 (V / V / V...
example 2
[0037] A kind of chiral chromatographic separation analysis method of dihydroquercetin enantiomer is as follows:
[0038] (1) Instruments and conditions: High performance liquid chromatography: Agilent 1200 series liquid chromatography
[0039] Chromatographic column: the specification is 250×4.6mm; the column is filled with amylose (3,5-dimethylphenylcarbamate) chiral stationary phase produced by Guangzhou Yanchuang Biotechnology Development Co., Ltd., namely Y3.
[0040] Mobile phase: n-hexane: trifluoroacetic acid: ethanol = 80:0.1:20 (V / V / V)
[0041] Flow rate: 1.0mL / min
[0042] Column temperature: 25°C
[0043] Injection volume: 10μL
[0044] Detection wavelength: 290mm
[0045] (2) Implementation
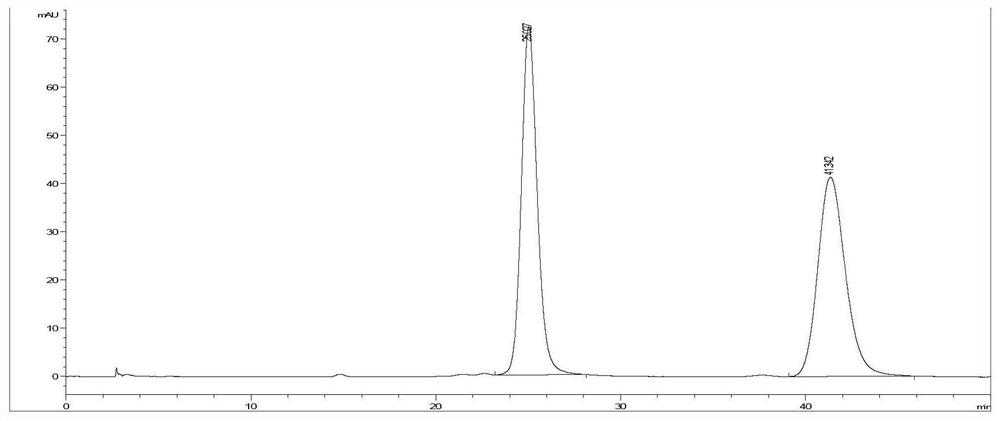
[0046] Dihydroquercetin (purity is 99%) is formulated into 0.5mg / mL solution with ethanol, on Agilent 1200 series high-performance liquid chromatograph, adopt Y3 chromatographic column (250×4.6mm), the mobile phase is hexane:trifluoroacetic acid:ethanol=80:0.1:20 (V / ...
example 3
[0048]A kind of chiral chromatographic separation analysis method of dihydroquercetin enantiomer is as follows:
[0049] (1) Instruments and conditions: High performance liquid chromatography: Agilent 1200 series liquid chromatography
[0050] Chromatographic column: the specification is 250×4.6mm; the column is filled with cellulose-tris(3-chloro-4-methylphenylcarbamate) chiral stationary phase produced by Guangzhou Yanchuang Biotechnology Development Co., Ltd., namely Y5.
[0051] Mobile phase: n-hexane: trifluoroacetic acid: ethanol = 80:0.1:20 (V / V / V)
[0052] Flow rate: 1.0mL / min
[0053] Column temperature: 25°C
[0054] Injection volume: 10μL
[0055] Detection wavelength: 290mm
[0056] (2) Implementation
[0057] Dihydroquercetin (purity is 99%) is formulated into 0.5mg / mL solution with ethanol, on Agilent 1200 series high-performance liquid chromatograph, adopt Y5 chromatographic column (250×4.6mm), the mobile phase is n-hexane: trifluoroacetic acid: ethanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com