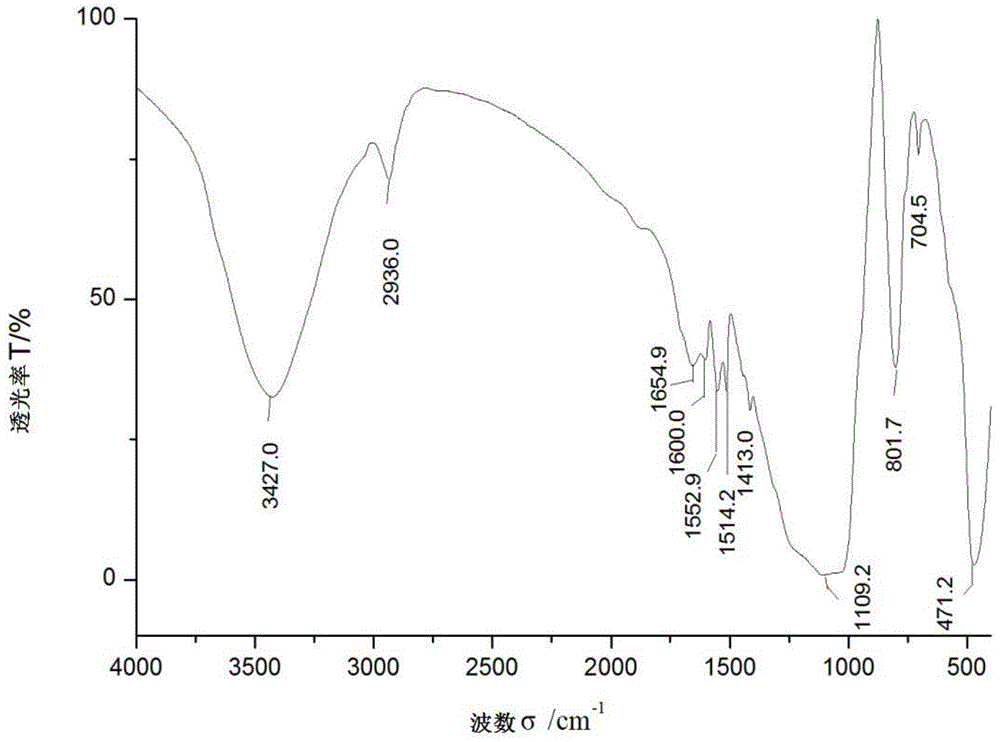
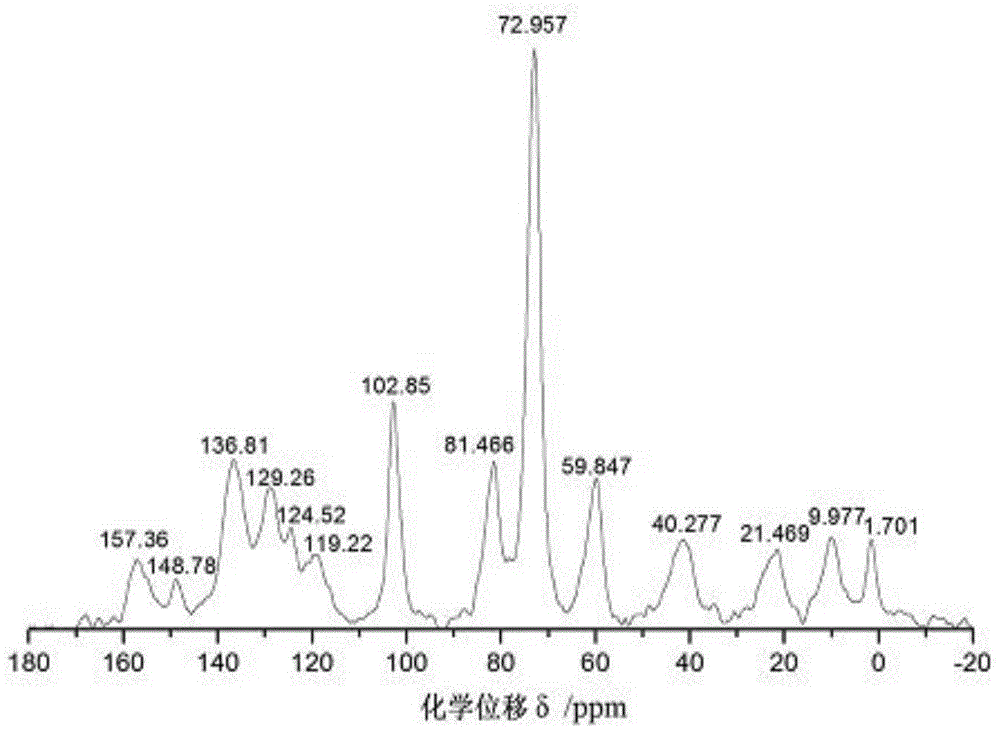
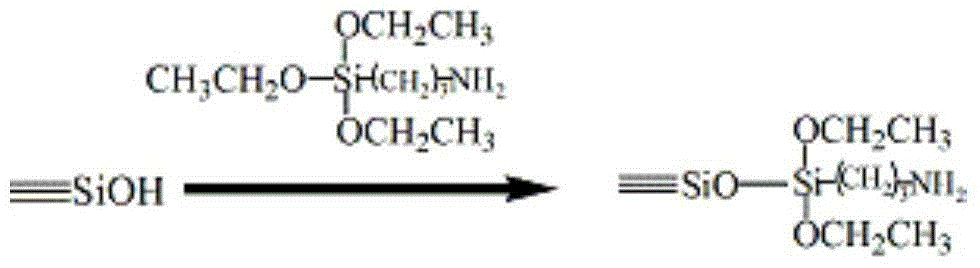
Preparation method for beta-cyclodextrin chiral stationary phase
A chiral stationary phase, cyclodextrin technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of difficult purification of the reaction, tailing of high performance liquid chromatography peaks, shortened column life, etc. Elution, Ease of Derivatization, Ease of Operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 7.5g of activated silica gel and 70mL of anhydrous toluene into a 100mL three-necked flask, heat and reflux with a water separator to remove water for 0.5h, then add 4.2mL of 3-aminopropyltriethoxysilane dropwise, and at 100°C The reaction was stirred for 4h. After cooling to room temperature and washing with anhydrous toluene for 5 times, 2.3 mL of trimethylchlorosilane was added dropwise, and the reaction was stirred at 50° C. for 4 h. Cool to room temperature, wash 5 times with anhydrous toluene, take samples and make elemental analysis. Calculate and weigh 3.0g 4,4'-diphenylmethane diisocyanate and add it to the reaction system, 2 , stirred and reacted at 80°C for 6h, cooled to room temperature, and washed 5 times with anhydrous toluene. Calculate and weigh 5.5g β-CD and add it to the reaction system, feed N 2 , Stir the reaction at 80°C for 8h. After cooling to room temperature, it was washed twice with anhydrous toluene, twice with acetone, and three times...
Embodiment 2
[0042] Add 5g of activated silica gel and 70mL of anhydrous toluene into a 100mL three-necked flask, heat and reflux with a water separator and stir to remove water for 0.5h, then add 3.4mL of 3-aminopropyltriethoxysilane dropwise, and stir at 100°C Reaction 4h. After cooling to room temperature and washing with anhydrous toluene for 5 times, 1.8 mL of trimethylchlorosilane was added dropwise, and the reaction was stirred at 50° C. for 4 h. Cool to room temperature, wash 5 times with anhydrous toluene, take samples and make elemental analysis. Calculate and weigh 1.1g 4,4'-diphenylmethane diisocyanate and join in the reaction system, pass into N 2 , stirred and reacted at 80°C for 6h, cooled to room temperature, and washed 5 times with anhydrous toluene. Calculate and weigh 3.9g β-CD and add it to the reaction system, feed N 2 , Stir the reaction at 80°C for 8h. After cooling to room temperature, it was washed twice with anhydrous toluene, twice with acetone, and three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com